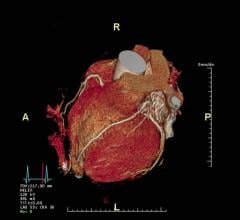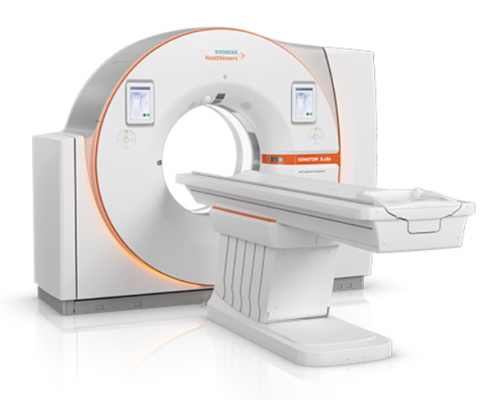
November 21, 2019 — The Food and Drug Administration (FDA) has cleared the new Somatom X.cite premium single-source computed tomography (CT) scanner from Siemens Healthineers together with the new myExam Companion intelligent user interface concept. The intuitive user interface of the myExam Companion guides the user through the exam workflow with precise questions about the patient, potentially enabling optimization of the state-of-the-art features of a premium CT scanner such as the Somatom X.cite. The myExam Companion combines available patient data such as gender and age with other user or machine-observable, patient-specific information – for example, a patient’s electrocardiogram (ECG) waveform for a cardiac study – to identify optimal acquisition and reconstruction techniques for each patient. Together, these innovations make the imaging process more efficient, compensating for differences in user experience levels and scan complexities on even the most complicated acquisitions, including coronary CT angiography (CTA) studies.
The first CT scanner launching with the myExam Companion intelligent user interface concept, the Somatom X.cite uses the Vectron X-ray tube, which was previously available only on the Somatom Force dual source CT scanner. In combination with a large 82cm gantry bore, the Somatom X.cite offers advanced imaging capabilities with an even greater focus on patient comfort.
myExam Companion
The myExam Companion enables technologists to utilize the full potential of not only the Somatom X.cite, but also the scanners of the Somatom go. CT platform.¹ Siemens Healthineers analyzed nearly 200,000 scan protocols using artificial intelligence and tapped clinical experts to identify key questions regarding the framework conditions for an optimized scan result. On this basis, Siemens Healthineers designed scan strategies and clinical decision trees to aid in proper setup and scanner operation. At its core, the myExam Companion renders complicated clinical decision-making into these 20-plus, user-definable clinical decision trees that are displayed at the human-machine interface, either at the operator console or using the scanner’s mobile, detachable tablets. These clinical decision tree workflows are sharable across the institution.
"Lack of staff and time and the creation of standards are some of the biggest hurdles in the daily business of radiology,” says André Hartung, president of Diagnostic Imaging at Siemens Healthineers. “With the SOMATOM X.cite and the myExam Companion, we provide our customers with unique tools to overcome these hurdles effectively. It is a major step for our imaging portfolio into the world of intelligent user support and for Siemens Healthineers on our way to becoming a leader in clinical decision support."
Somatom X.cite
In addition to its large, 82 cm gantry bore and optional Moodlight feature, the Somatom X.cite includes the Patient Observation Camera. This new 2-D visualization camera allows the technologist to closely monitor the patient while he or she is inside the gantry for potentially fewer patient motion artifacts. The Somatom X.cite also includes Visual Patient Instruction, a new visual breath hold indicator on the gantry that guides the patient through the breath hold process with color-guided visual instructions.
Together, these features create a holistic, patient-centric scanning experience.
The technologist can control scan preparation and oversee the scan via a detachable, gantry-mounted tablet, enabling the technologist to remain with the patient until immediately before the scan. An optional FAST Integrated Workflow with FAST 3D Positioning Camera collects further anatomical information and automatically positions the patient at isocenter. The Vectron X-ray tube enables a maximum 1,200 mA High Power mode at low 70 or 80 kV stations; low kV scanning with significant power reserves enables lowering of radiation dose and contrast media volumes. Together with the Stellar Infinity detector, the Vectron tube enables visualization of even the smallest details with 0.30 mm precision resolution. To further personalize the scanning process, the Somatom X.cite is capable of fine-tuned 10 kV steps, enabling a greater range of personalized dose modulation.
The Somatom X.cite CT scanner with the myExam Companion intelligent user interface concept will be displayed at the Siemens Healthineers booth (7530) at the 105th Scientific Assembly and Annual Meeting of the Radiological Society of North America (RSNA), Dec. 1-7 at McCormick Place in Chicago.
¹ The SOMATOM.go systems with my Exam Companion are pending 510(k) clearance, and are not yet commercially available in the United States.
For more information: http://siemens-healthineers.us/somatom-xcite


 April 22, 2024
April 22, 2024