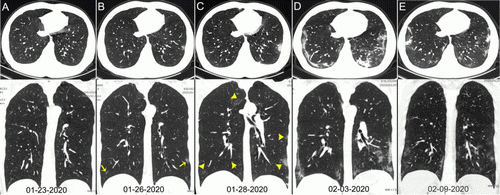
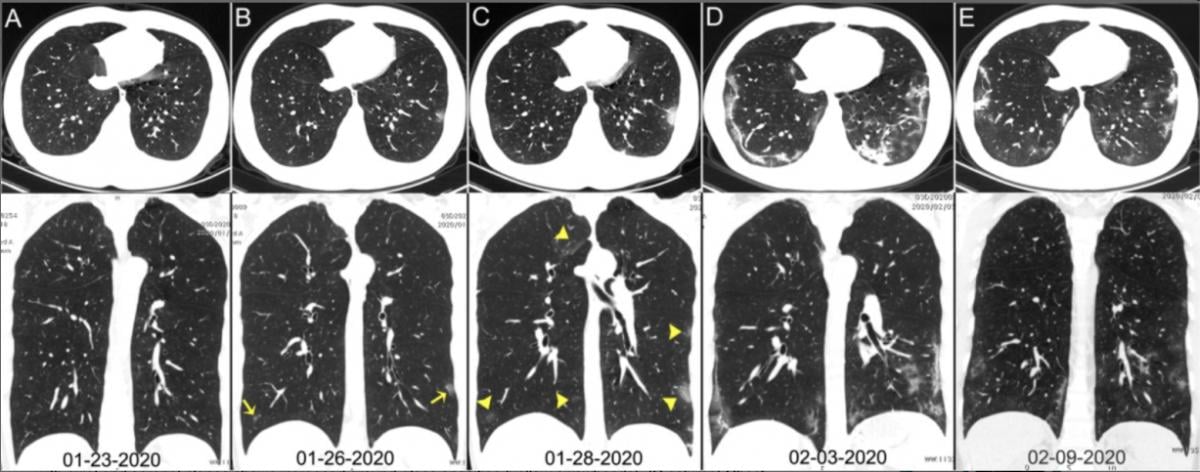
Chest CT images of a 29-year-old man with fever for 6 days. RT-PCR assay for the SARS-CoV-2 using a swab sample was performed on Feb. 5, 2020, with a positive result. (A column) Normal chest CT with axial and coronal planes was obtained at the onset. (B column) Chest CT with axial and coronal planes shows minimal ground-glass opacities in the bilateral lower lung lobes (yellow arrows). (C column) Chest CT with axial and coronal planes shows increased ground-glass opacities (yellow arrowheads). (D column) Chest CT with axial and coronal planes shows the progression of pneumonia with mixed ground-glass opacities and linear opacities in the subpleural area. (E column) Chest CT with axial and coronal planes shows the absorption of both ground-glass opacities and organizing pneumonia. Image courtesy of Radiology
February 26, 2020 — In a study of more than 1,000 patients published in the journal Radiology, chest CT outperformed lab testing in the diagnosis of 2019 novel coronavirus disease (COVID-19). The researchers concluded that CT should be used as the primary screening tool for COVID-19 (also called SARS‐CoV‐2).[1]
In the absence of specific therapeutic drugs or vaccines for COVID-19, it is essential to detect the disease at an early stage and immediately isolate an infected patient from the healthy population.
According to the latest guidelines published by the Chinese government, the diagnosis of COVID-19 must be confirmed by reverse-transcription polymerase chain reaction (RT-PCR) or gene sequencing for respiratory or blood specimens, as the key indicator for hospitalization. However, with limitations of sample collection and transportation, as well as kit performance, the total positive rate of RT-PCR for throat swab samples has been reported to be about 30 to 60 percent at initial presentation.
In the United States, similar RT-PCR test kits used by the Centers for Disease Contriol and Prevention (CDC) to test patients and confirm coronavirus infections were cleared for clinical use in the U.S. by an emergency approval by the U.S. Food and Drug Adminsitration (FDA) Jan. 30. However, when these kits were sent to states, local authorities performed verification testing for quality control and found the tests are not 100 percent accurate, and showed some false negative results.
"As we’ve pushed tests out to the state, they did what we would expect as part of the normal procedures, which is do the verification in their own laboratories. There were problems identified with the test kits. That is a normal part, unfortunately, of these processes. We obviously would not want to use anything but the most perfect possible kits," said Nancy Messonnier, M.D., the director for the National Center for Immunization and Respiratory Diseases at the CDC.
She said the CDC is working with the FDA to resolve the issues. However, the CDC still considers it a priority to get the kits out to patients as soon as possible. CT might offer an alternative way to help diagnose COVID-19 positive patients.
In the current public health emergency, the low sensitivity of RT-PCR implies that a large number of COVID-19 patients would not be identified quickly and may not receive appropriate treatment or isolation. In addition, given the highly contagious nature of the virus, they carry a risk of infecting a larger population.
CT Imaging May Offer Better Sesitivity Than RT-PCR Testing for Coronavirus
“Early diagnosis of COVID-19 is crucial for disease treatment and control. Compared to RT-PCR, chest CT imaging may be a more reliable, practical and rapid method to diagnose and assess COVID-19, especially in the epidemic area,” the Radiology study authors wrote.
Chest CT, a routine imaging tool for pneumonia diagnosis, is fast and relatively easy to perform. Recent research found that the sensitivity of CT for COVID-19 infection was 98 percent compared to RT-PCR testing sensitivity of 71 percent.
For the current study, researchers at Tongji Hospital in Wuhan, China, set out to investigate the diagnostic value and consistency of chest CT imaging in comparison to RT-PCR assay in COVID-19.
Included in the study were 1,014 patients who underwent both chest CT and RT-PCR tests between January 6 and February 6, 2020. With RT-PCR as reference standard, the performance of chest CT in diagnosing COVID-19 was assessed. For patients with multiple RT-PCR assays, the dynamic conversion of RT-PCR test results (negative to positive, and positive to negative, respectively) was also analyzed as compared with serial chest CT scans.
The results showed that 601 patients (59 percent) had positive RT-PCR results, and 888 (88 percent) had positive chest CT scans. The sensitivity of chest CT in suggesting COVID-19 was 97 percent, based on positive RT-PCR results. In patients with negative RT-PCR results, 75 percent (308 of 413 patients) had positive chest CT findings. Of these, 48 percent were considered as highly likely cases, with 33% as probable cases. By analysis of serial RT-PCR assays and CT scans, the interval between the initial negative to positive RT-PCR results was 4 to 8 days.
“About 81 percent of the patients with negative RT-PCR results but positive chest CT scans were re-classified as highly likely or probable cases with COVID-19, by the comprehensive analysis of clinical symptoms, typical CT manifestations and dynamic CT follow-ups,” the authors wrote.
A couple early CT imaging patient case studies coming out of China in the past month reported COVID-19 was premilinarly diagnosed from CT scans in several patients before they started to show positive RT-PCR test results.
Additional COVID-19 radiology research can be found at Special Focus: COVID-19.
Chest CT images of a 29-year-old man with fever for 6 days. RT-PCR assay for the SARS-CoV-2 using a swab sample was performed on Feb. 5, 2020, with a positive result. (A column) Normal chest CT with axial and coronal planes was obtained at the onset. (B column) Chest CT with axial and coronal planes shows minimal ground-glass opacities in the bilateral lower lung lobes (yellow arrows). (C column) Chest CT with axial and coronal planes shows increased ground-glass opacities (yellow arrowheads). (D column) Chest CT with axial and coronal planes shows the progression of pneumonia with mixed ground-glass opacities and linear opacities in the subpleural area. (E column) Chest CT with axial and coronal planes shows the absorption of both ground-glass opacities and organizing pneumonia. Image courtesy of Radiology
CDC Said it is a Question of When, Not If Coronavirus Will Spread in the U.S.
Messonnier said the goal of the CDC is to prevent the spread of COVID-19 as long as possible so the country's healthcare system can prepare for its potentially inevitable arrival. However, she said it is not possible to catch all the cases of COVID-19.
"We never expected we’d catch every traveler with novel coronavirus from China. It would be impossible," Messonnier explained. "We’re not seeing spread here in the United States yet, but it is possible, even likely, that it may eventually happen. Our goal continues to be slowing the introduction of the virus into the U.S. This buys us more time to prepare our communities for more cases and possibly sustained spread."
She said this new virus represents a tremendous public health threat. "We don’t yet have a vaccine for this novel virus, nor do we have a medicine to treat it specifically. We are taking and will continue to take aggressive action to reduce the impact of this virus, and that it will have on the communities in the U.S.," Messonnier said.
The CDC is working with state, local and territorial health departments to ready the public health workforce to respond to local cases and the possibility this outbreak could become a pandemic.
Messonnier said the CDC has a contingency plan for large influenza pandemics, which the CDC is adapting for a COVID-19 outbreak. She said it is very informative in terms of what people can expect in the coming weeks if the virus starts spreading in our community.
The document is a Morbidity and Mortality Weekly Report (MMWR) recommendations and report titled “Community Mitigation Guidelines to Prevent Pandemic Influenza, United States – 2017.”
"These materials will serve as a blueprint for the community interventions we will use here in the U.S.," Messonnier explained. "If you’re watching the news, you may be hearing about schools shutting down and businesses closing in countries in Asia to reduce the potential spread of this virus. The day may come where we need to implement such measures in the U.S. communities."
Read more about the CDC announcement.
Additional Coronavirus Resources for Clinicians:
• World Health Organization (WHO) COVID-19 situation reports.
• World Health Organization (WHO) coronavirus information page
• U.S. Food and Drug Administration (FDA) COVID-19 information page
• Centers for Disease Control (CDC) COVID-19 information page
Related Coronavirus Content:
The Cardiac Implications of Novel Coronavirus
Radiologists Describe Coronavirus CT Imaging Features
Coronavirus Update from the FDA
CT Imaging of the 2019 Novel Coronavirus (2019-nCoV) Pneumonia
Infervision in the Frontlines Against the Coronavirus
CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV)
Chest CT Findings of Patients Infected With Novel Coronavirus 2019-nCoV Pneumonia
Find more related clinical content Coronavirus (COVID-19)
FDA Resource on the Novel coronavirus (COVID-19)
Mount Sinai Physicians the First in U.S. Analyzing Lung Disease in Coronavirus Patients from China
Reference:

 April 17, 2024
April 17, 2024