BLOG: Cloud-Based Imaging Platform Streamlines Workflows, Ensures Data De-Identification and Improves Collaboration in Clinical Trials
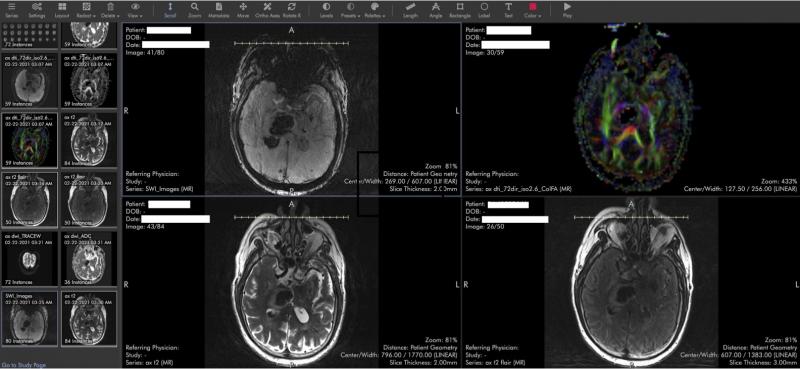
Stroke imaging using Ambra health’s in built viewer.
Medical imaging, an often-overlooked piece of the puzzle in establishing a holistic patient health record, plays a fundamental role in the design of research studies and clinical trials. Imaging workflows, however, can present various challenges because imaging traditionally has been exchanged on CDs that are uploaded and mailed. From obtaining, identifying and uploading the images, to reading, analyzing and archiving them correctly — the entire process is error-prone and often delays progress in trials due to inconsistent or slow imaging data input.
In addition, imaging is also a large component of potential privacy breaches as studies require sharing electronic health record data across multiple environments. The ability to collect and organize huge amounts of data and securely remove patient identifiers through de-identification and anonymization strategies are paramount in research studies to safeguarding patient privacy.
University of Cincinnati, which brings together top clinicians and researchers to provide world-class care, implemented a cloud-based PACS imaging platform from Ambra Health in their Radiology department to automate its process of medical imaging for clinical trials.
According to Achala Vagal, M.D., M.S., Professor of neuroradiology and vice chair of research in the Department of Radiology, University of Cincinnati College of Medicine, the cloud-based imaging solution has been in place since 2018 allowing for the secure upload, anonymization, performing clinical reads and gathering and exporting data from the clinical reads so that imaging data can be matched with clinical data.
“UC manages a lot of large clinical trials and we are the imaging core labs for many of these trials; they can be anywhere from six sites to 125 sites,” Vagal said. “There are a lot of imaging studies that come from these different sites. Thus, data need to be managed well and anonymization is a very big part of what we do. The Ambra cloud PACS has done a great job when it comes to managing and customizing the workflow because no trial is designed in the same way.”
Before the implementation of this platform, the gathering of radiological data at UC could take from several weeks to up to months, according to Vivek Khandwala, Ph.D., manager for the Radiology Services Imaging Core Lab, University of Cincinnati College of Medicine.
“The entire timeline would take close to two to three months because site coordinators would have to put in a request for the imaging studies, radiology departments would have to burn discs, discs would need to be mailed and QA/QC at UC. If an error is discovered the whole process would need to be repeated,” Khandwala explained. “With Ambra, there is a capability of sending images directly from the scanner to the cloud; or be web uploaded. So, the turnaround time has gone down from a couple of months to less than a few days, which is phenomenal.”
With research, the process of anonymizing and de-identification can be dangerously erroneous.
“As a society, we are getting more and more cognizant of data privacy and imaging is nothing different. When you think of a cloud-based system, which can be accessed anywhere and there are multiple users, one of the biggest challenges is the ability to carefully de-identify all images so they cannot be tracked back to a particular patient,” Vagal said.
Ambra’s automation removes DICOM tags on the client-side before the study leaves the sending facility, eliminating the risk of accidentally leaving patient information tags in place. Ambra also has a pixel de-identification tool to even remove identifiable information from the DICOM itself.
In addition, the system allows for customization of timepoint fields, project users and roles, case report forms and trial workflows. Centrally managed and automated workflows enable studies to be routed to end destinations including local file directories, research repositories and third-party viewers or post-processing systems. Incoming studies from outside sites are routed through configurable workflows with automated sharing to organizations, locations, groups and users. Each project may have its own customizable electronic case report form (eCRF) that allows the gathering of radiological data that later can be exported to the statistical analysis centers.
“Pre-Ambra’s cloud-based solution, if you wanted to look at a case, a coordinator would have to bring your disc to the investigator/radiologist. They would have to put it into a CD drive and load it up. Then, they would have to open a second image viewing software to be able to review those images. They would have to fill out the study case report form in a different place. With this cloud-based system, using just an internet connection, you can log into the website, you do not need any data being moved, and you can view the images and fill out the form all in one platform, and best part is that you could be sitting on a beach doing it,” Khandwala said.
Being cloud-based also has helped UC continue its research seamlessly and improve collaboration especially during COVID. “All we need is a good internet connection. There is no physical CD, CD drives or touchpoints; it is all online. As a department and as an imaging research lab, we have been extremely productive, even during COVID,” Vagal added.
Editor’s Note: This is the fourth blog in a series about sharing patient images. The first BLOG: Why Image Transfer Needs To Be Electronic, can be found here. The second BLOG: How Artificial Intelligence Can Help In Image Transmission of Images, can be found here. The third BLOG: A Patient First Approach to Improving Imaging Accessibility and Usability can be found here.




 April 13, 2024
April 13, 2024