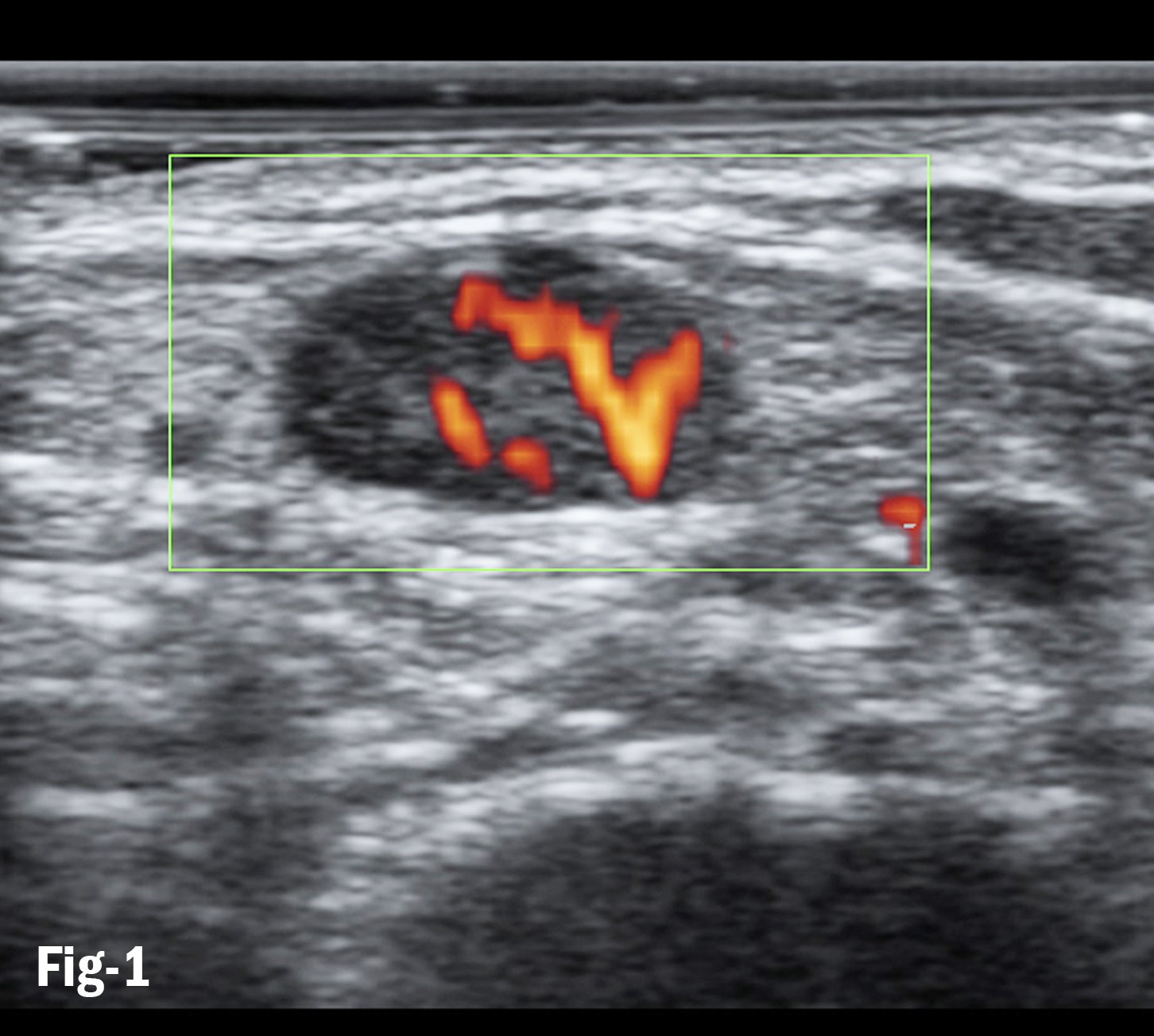
Figure 1. Doppler flows in subpleural consolidation shows smoothly dilated branching arteries
COVID-19 is routinely studied using lung ultrasound and quantifying the disease by the “white lung” appearance of multiple B-lines targeting the pulmonary parenchyma. All modern sonographic equipment offers color Doppler features, which have not been fully utilized in the differential diagnosis of inflammatory and malignant lesions. In addition to the sonographic characteristics of the B-mode image, the type of vascularization is important for clinical assessment of a lesion in terms of differential diagnosis. In sonographic examinations, the well-established procedures of power or color Doppler sonography and contrast enhanced ultrasonography (CEUS) are used.
Recently color-Doppler sonography characteristics of bacterial pneumonia, obstructive atelectasis, lung infiltrates and bronchial carcinoma have been described. Preliminary studies show that CEUS can be performed on the chest in some clinical settings. Various diseases of the lung are characterized by specific contrast sonography findings. The purpose is to describe color/power Doppler sonography and CEUS findings in the presence of peripheral lung consolidations.
Pathophysiology
The lung is supplied by dual vascularization. Perfusion is achieved on the one hand by lung circulation responsible for pulmonary gas exchange. Lung circulation is accomplished by the pulmonary arteries and their ramifications as well as venules and lung veins. The lung itself is nourished by the bronchial arteries. In contrast to systemic circulation, circulation by the pulmonary arteries is characterized by elastic arteries and their initial branches. The downstream arteries have inelastic muscular walls and from the arterioles onward are partly muscular precapillaries. In contrast to systemic circulation in which arterioles are the main vessels of resistance, in lung circulation the resistance is equally distributed between arteries, capillaries and veins. Flow in the pulmonary arteries and capillaries is pulsatile and not continuous. In contrast to hypoxic vasodilatation in the systemic circulation, hypoxic vasoconstriction occurs in lung circulation. When lung tissue is affected by a malignant tumor, the carcinoma invades the pulmonary arteries of the affected lung segment where the center of the tumor shows irregular and tortuous vascular patterns.
Vascularization is reduced by stenosis, shunting, arteriovenous fistulae and occlusion of the pulmonary arteries. As a rule, the bronchial arteries originate on the left side from the aortic arch and on the right side from the intercostal artery forming a vascular ring at the hilum of the lung. Anastomoses (blocked arteries) between the two systems that are normally closed become open owing to hypoxia and blood supply is provided by bronchial arteries. Angiographic studies have shown that peripheral lung diseases at the pleural wall, such as benign cavitary lesions, lung cysts, lung abscesses and liquefying pneumonia, are nourished by bronchial arteries.1 However, malignant primary lung tumors and lung metastases are vascularized by bronchial arteries. The intercostal arteries originate from the thoracic aorta and run a strictly intercostal course in the chest wall along the ribs. They are the only vessels visualized on sonography, even in healthy volunteers. Particularly in cases of lesions in the chest wall, these vessels play an important role in tumor vascularization since neo-angiogenesis of primary bronchial carcinomas mainly originates in the bronchial arteries. The use of contrast-assisted sonography allows microvessels whose width is larger than that of capillaries to be visualized.2
Contrast-Assisted Sonography
Similar to power, angio, B-flow or color Doppler sonography, lung infarctions/lung hemorrhages are marked by the absence of contrast enhancement on contrast-assisted sonography. Contrast-assisted sonography (CEUS) allows the investigator to make a reliable distinction between vascularized and nonvascularized peripheral lung tissue differentiating infarct from compressive atelectasis due to pleural effusion.
Pleurisy
Color Doppler sonography. The appearance of pleurisy on the B-mode image is similar to that of a lung infarction. Depending on the size of the invasion, which is transformed into pleural pneumonia, the qualitative color Doppler sonography characteristically shows pronounced vessels with predominant evidence of an arterial high-impedance flow profile on spectral analysis such as that seen in branches of the pulmonary artery.
Contrast-assisted sonography. In keeping with color-Doppler sonography findings, pleurisy takes very little time for contrast enhancement to start and is marked by strong contrast enhancement on contrast-assisted sonography. This is indicative of primary vascularization through pulmonary arteries.3 The value of contrast-assisted sonography lies in its potential for differential diagnosis of nonvascularized peripheral lung lesions such as lung infarction, malignant lesions or scar tissue.
Color Doppler sonography. A peripheral round lesion of the lung at the wall of the pleura occurring as the cardinal symptom may be due to a benign or malignant lesion. Of decisive importance is the fact that, independent of the cause, the evidence of flow signals is dependent on the size of the lesion. On careful investigation one frequently finds arterial high-impedance flow signals from pulmonary arteries and low-impedance flow signals from bronchial arteries in benign as well as malignant peripheral lung lesions. Power or color Doppler sonography is not currently accepted for distinguishing between benign and malignant peripheral round lesions.
In keeping with the variable findings on color Doppler sonography, CEUS also shows a heterogeneous pattern of vascularization. Malignant lesions — whether lung metastases or peripheral bronchial carcinomas — are marked by delayed start of contrast enhancement and reduced extent of contrast enhancement. This is indicative of predominant vascularization through bronchial arteries. Depending on the underlying structure, however, lung metastases of renal cell carcinomas and frequently also metastases of malignant lymphoma show pronounced contrast enhancement, as a sign of strong tumor angiogenesis. The extent and homogeneity of tumor angiogenesis depend, among other factors, on tumor size. In some cases, contrast enhanced ultrasound is useful to assess the malignant or benign nature of the lesion.
Large Lung Consolidation
Pneumonia is seen on X-rays and sonography in conjunction with the principal finding of a peripheral lung consolidation at the pleural wall. On B-mode image sonography, partial pneumonia demonstrates pronounced air bronchograms while complete consolidation is seen as so-called lung hepatization. On color Doppler sonography, pneumonia is marked by significantly ramified vessels that correspond to segmental branches of the pulmonary artery. Here also, owing to hypoxia one frequently finds an arterial monophasic flow signal of central bronchial arteries in the invaded lung tissue.
In principle it should be noted that certain subtypes of adenocarcinoma may appear similar to pneumonia on the B-mode image and on color Doppler sonography as well. Depending on the extent of hypoxic vasoconstriction in the pulmonary artery, the peripheral vascular tree of pulmonary arteries may not be visualized on qualitative color Doppler sonography in the presence of advanced pneumonia. Bronchial arteries react to hypoxia by developing vasodilatation, as do all other arteries in the body. This explains the different resistance indices of pneumonia and atelectasis. Thus, in the presence of lobar pneumonia parallel to the pulmonary arteries one occasionally finds an arterial monophasic flow pattern with low resistance indices, indicative of central bronchial arteries.
The tuberculous infiltrate is a special phenomenon. It is characterized by marked vessels on color Doppler sonography in terms of qualitative findings. On spectral analysis, however, it is seen as a monophasic curve, corresponding to bronchial arteries. Cavitary lesions such as tuberculosis, liquefactions, necrosis, abscesses and pseudocysts are characterized by the presence of predominant vascularization through the bronchial artery in the marginal areas around the lesion.
Contrast-assisted sonography. In keeping with the findings on color Doppler sonography, classic pneumonia is characterized by a short period until the start of contrast enhancement and strong contrast enhancement on contrast-assisted sonography. This is indicative of predominant vascularization though pulmonary arteries. Reduced contrast enhancement is observed in cases of lobar pneumonia and can be explained by hypoxic vasoconstriction of the pulmonary artery. Delayed contrast enhancement indicates vascularization by the bronchial artery and is observed in cases of liquefaction and chronic pneumonia. Such avascular areas in the region of pneumonia can be clearly demarcated on contrast-assisted sonography. Inhomogeneous contrast enhancement is helpful in identifying typical courses of disease with evidence of consolidation, necrosis, infarct areas, inward bleeding, or abscesses. Especially in parapneumonic echogenic effusions with multiple septa and a suspected pleural empyema, the area can be clearly demarcated from the infiltrated lung.4
Large Lung Consolidation: Compressive Atelectasis
Color Doppler sonography. Compressive atelectasis is seen on radiographs and sonography in conjunction with the cardinal finding of a peripheral basal lung consolidation at the pleural wall. The main finding is a pleural effusion, followed by the visualization of compressed lung tissue. On qualitative color Doppler sonography atelectasis is seen as a strongly ramified vessel. On arterial spectral analysis one usually finds a high impedance flow signal indicative of pulmonary arteries.
Contrast-assisted sonography. In concurrence with color Doppler sonography findings, compressive atelectasis is characterized by a brief period until the start of contrast enhancement and strong contrast enhancement on contrast-assisted sonography. This is indicative of vascularization exclusively through the pulmonary arteries. The contrast assisted sonography pattern of compressive atelectasis is very specific. Round lesions in atelectatic lung tissue are marked by poor contrast enhancement.
Large Lung Consolidation: Obstructive Atelectasis Color Doppler Sonography
The obstructive lung atelectasis is seen as a largely homogeneous hypoechoic transformation on the B-mode image. Depending on the duration of the obstruction, evidence of a “fluid bronchogram” is a characteristic feature in this setting. On qualitative color Doppler sonography, one finds pronounced vessels with evidence of an arterial high impedance flow signal due to the branches of the pulmonary artery and arterial monophasic flow profile of central bronchial arteries in atelectatic tissue as a result of hypoxia. A frequent finding is the central tumor underlying the atelectasis well imaged since the consolidated atelectatic lung tissue serves as an “acoustic window” to explore central lung structures. This disturbed vascular architecture of the pulmonary artery appears on sonography as reduced or no visualization of vessels in the atelectatic lung tissue.5
Contrast-assisted Sonography
In keeping with the color Doppler sonography findings, a recent obstructive atelectasis is marked by the same features as compressive atelectasis — a short period of time until contrast enhancement and strong contrast enhancement on contrast-assisted sonography. This is indicative of atelectatic lung tissue being entirely vascularized by the pulmonary arteries. In this phase, in patients with a central tumor formation, the tumor may be demarcated from atelectatic lung tissue more clearly by contrast-assisted sonography than by B-mode sonography. In cases of obstruction of longer duration, within the atelectasis one may find liquefactions and abscesses. These potential lesions as well as metastases in atelectatic lung tissue can be reliably diagnosed by contrast-assisted sonography.
In the course of tumor-related obstructive atelectasis, depending on the structure of the tumor there may be infiltration and occlusion of pulmonary arteries. In this situation contrast-assisted sonography shows delayed start of contrast enhancement and reduced contrast enhancement. This is indicative of a switch to vascularization of atelectatic lung tissue by bronchial arteries. In general, the contrast-assisted sonography pattern in cases of obstructive atelectasis is heterogeneous. The pattern of compression atelectasis on contrast enhanced ultrasonography is very specific. In atelectatic lung tissue, round lesions indicative of lung metastases, and wedge-shaped peripheral defects as a sign of infarction, reveal poor or no contrast enhancement. In cases of chronic compression atelectasis, pulmonary artery vascularization may be transformed into purely nutritive bronchial artery vascularization. Contrast-enhanced ultrasound can be very valuable in clarifying the etiology of a pleural effusion. An echogenic effusion with multiple septa can be clearly distinguished from tumor tissue and tumor tissue along the pleura from a hematoma or fibrinous tissue.5
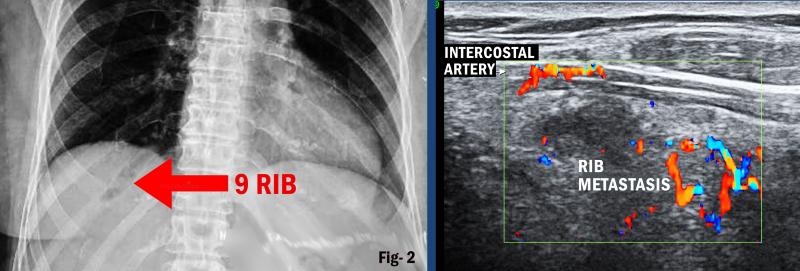
Space-occupying Lesion of the Chest Wall: Color Doppler Sonography
Sonography is the method of choice to explore the chest wall. The intercostal arteries supplying the chest wall are usually seen even in healthy individuals by the use of color Doppler sonography. Tumors in the chest wall or pleural metastases are characterized by predominantly intercostal vascularization with a monophasic flow profile when the lesions are adherent to the chest wall. When the tumor has invaded the lung, color Doppler sonography may show different flow signals as a sign of complex arterial tumor vascularization. The extent of contrast enhancement of the arterial phase may vary. Tumors with pronounced neovascularization reveal strong contrast enhancement. Contrast-enhanced ultrasound is very important for the delineation of non-vascularized lesions such as hematomas or abscesses.6
Quantifiable Digital Scanning and Image Guided Interventions
4-D imaging is real-time observation of a 3-D data set that permits image guided biopsy of the most virulent area of the infiltrate or tumor, targets the densest area of B-lines or neovascularity and allows the pathologist to focus on the most suspicious region of the lymph node mass excised during surgery. Similarly, during real time exam the fluid content of a pleural complex cyst maybe aspirated under better visual control and perilesional vessels avoided in a timely manner. This feature enhances the reliability of image guided treatments and fusion of this data set with computed tomography (CT) or magnetic resonance imaging (MRI) scans.
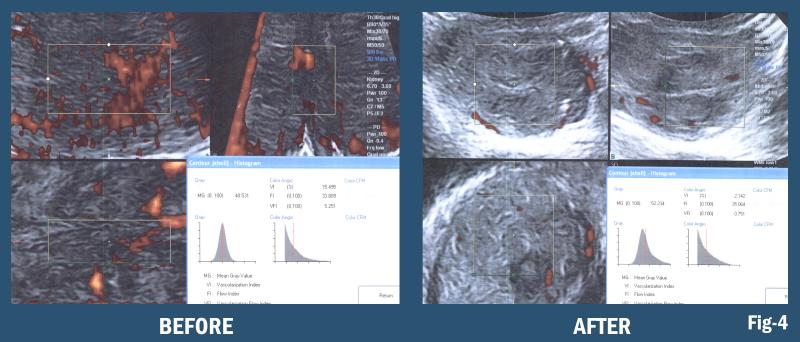
3-D/4-D Doppler Histogram
In addition to the frequent use of puncture for pleural effusion, space-occupying masses accessible to sonographic investigation, located in the chest wall, pleura, lung or anterior mediastinum, are important indications. Depending on their topographical position and the diagnostic availability and expertise, pathological changes not detectable by a transthoracic approach may be identified diagnostically by one of the interventional procedures displayed in the list in the previous section.7
Interventions in the thorax- indications:
1. Space-occupying mass in the thoracic wall (tumors, abscesses, hematomas, changes in the skeletal parts)
2. Space-occupying masses in the pleura
3. Pleural effusion and pleural empyema (very small quantities, loculated effusions)
4. Peripheral lung consolidations (lung tumor, pneumonia, lung abscess)
5. Mediastinal processes (anterior mediastinum)
Because of the potential risk of complications, the indication for the procedure should be established with care considering the probable comorbidities of the COVID-19 patient. Any sonographically demonstrable space occupying lesion can be punctured in principle. In a patient who is operable, a suspected malignant tumor located in the periphery will normally not be punctured, but will be resected as a first-line measure. Contraindications assuming acceptable coagulation parameters depend on the position of the mass relative to major structures and the invasiveness of the intervention. Urgent interventions require individual assessment of risk.
Bullous pulmonary emphysema and pulmonary hypertension are relative contraindications. When respiratory function is severely restricted or blood gas values are poor, a puncture should only be performed when the patient’s condition is expected to be improved by the therapeutic intervention. High-risk puncture sites should be avoided. Sonography-guided, CT-guided or fusion with CT and MRI are now possible at the bedside with the portable sonographic technologies. The addition of Doppler pre-procedure vessel mapping further reduces blood loss and accidental vascular perforation.8
Summary
Qualitative color Doppler sonography shows varied and partly characteristic findings in the presence of various types of lung consolidations and is therefore a valuable adjunct to B-mode sonography with regard to the etiological classification of peripheral lung lesions. In keeping with the physiological dual vascularization of the lung by the pulmonary arteries and the bronchial arteries, on color Doppler sonography one can distinguish between arterial high-impedance spectral curves and arterial low-impedance spectral curves in consolidated lung tissue. The former are assigned to pulmonary arteries and the latter to bronchial arteries.
Within peripheral lung consolidations, different entities show characteristic distribution patterns of pulmonary-artery and bronchial-artery flow signals in respect of frequency and location. Arterial spectral curve analysis is time-consuming. Reliable demarcation of vessels of tumor neo-angiogenesis is currently not possible with color Doppler sonography. Experience concerning contrast-assisted sonography in cases of peripheral lung lesions is limited. Contrast-assisted sonography at the chest can be performed easily and rapidly, and is therefore basically suitable for routine clinical use. Lung lesions can be distinguished by the time they acquire contrast until the start of contrast enhancement and the extent of contrast enhancement. Washout parameters are useful. Initial studies show that contrast assisted sonography at the chest can be helpful to differentiate ambiguous lung lesions and avoid operative complications.

Since 1974, Robert L. Bard, M.D., PC, DABR, FASLMS, has pioneered noninvasive digital imaging technologies as alternatives to radiation-producing diagnostic systems for evaluating solid organ neoplastic disease. He is internationally recognized as a leader in the field of 21st century 3-D ultrasonographic volumetric Doppler imaging.
References:
1. Bard R. Ed., Image Guided Treatment of COVID-19 Lung Disease. Springer (in press)
2. Mathis G, Chest Sonography. Springer 2017
3. Albrecht T, Blomley M, Bolondi L etal (2004) Guidelines for the use of contrast agents in ultrasound. Ultraschall Med 25:249–256
4. Babo HV, Müller KMG, Huzky A, Bosnjakovic-Buscher S (1979) Die Bronchialarteriographie bei Erkrankungen der Lunge. Radiologe 19:506–513
5. Civardi G, Fornari F, Cavanna L, Di Stasi M, Sbolli G et al (1993) Vascular signals from pleural-based lung lesions studied with pulsed doppler ultrasonography. JCU 21:617–622
6. Fissler-Eickhoff A, Müller KM (1994) Pathologie der Pulmonalarterien bei Lungentumoren. DMW 119:1415–1420
7. Forsberg F, Goldberg BB, Liu BB et al (1999) Tissue specific US contrast agent for evaluation of hepatic and splenic parenchyma. Radiology 210:125–132
8. Bard R (1970) Crescent sign in pulmonary hematoma. United States Air Force Medical Journal 1:11-13


 April 24, 2024
April 24, 2024