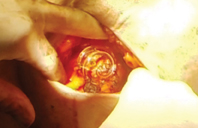
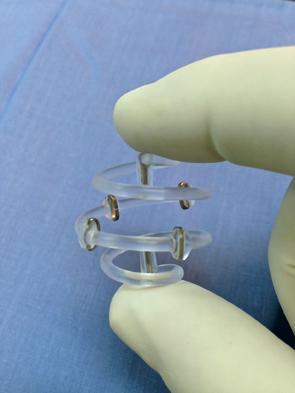
The spiral-shaped device is placed by a breast surgeon at the time of lumpectomy after the tumor and surrounding margins have been excised.
The trend in breast cancer treatment these days is all about precision, with the twin goals of controlling the cancer better and reducing side effects. Great advances have been made in radiation delivery methods that make it possible to more precisely target the tumor bed, plus a small margin around it where the cancer would be most likely to recur. By tightly focusing on these areas alone, radiation oncologists can minimize exposure to critical healthy tissue and structures such as the heart and lungs. Less damage to healthy tissue also means better cosmetic outcomes.
The problem is there’s more to precise radiation therapy than accurate delivery. Imagine a highly skilled baseball pitcher who can put a ball anywhere he wants in the strike zone — except his vision is sometimes a little blurry. Until very recently, that analogy fit the state of radiation therapy. You can’t precisely target radiation unless you can clearly view the target.
Why can’t radiation treatment planners always clearly see the target area in the patient’s body? It’s largely due to the limitations of traditional approaches to tissue marking of the target area. When the target area can’t be clearly visualized, radiation oncologists have to irradiate well beyond the surgical site to make sure the site and margins are included. This cautious approach, while appropriate to the circumstances, can put healthy tissue and structures at risk and makes it harder to get a satisfying cosmetic result.
Potential Solution Appears
Fortunately, a new 3-D marker has emerged that appears to address the problem. In my clinical experience and research with the device (BioZorb Tissue Marker, Focal Therapeutics Inc.), it directly addresses the current targeting issues. It also has additional advantages that other markers do not. Early users of the device have started accumulating evidence that documents its utility, and it is compatible with current advanced radiation therapy delivery methods such as multi-beam, 3-D conformal, intensity-modulated radiotherapy, image-guided radiotherapy, some forms of brachytherapy and volumetric modulated arc therapy.
The marker is a simple, but elegant, concept: titanium marker clips in a stable 3-D array accurately define the target space, remain clearly visible in a variety of situations and stay put over the long term. The device is spiral-shaped, with six permanent clips embedded along the spiral. Using standard techniques, it is placed by a breast surgeon at the time of lumpectomy after the tumor and surrounding margins have been excised. The marker clips define a 3-D area that is easily visible for treatment planners and radiation oncologists. I think of it as a glowing beacon that says, “Aim here.”
Because the marker clips stay in place, the 3-D marker accurately defines the target area for both the short and long term. The spiral is made of a bioabsorbable polymer. Over a period lasting a year or more, the patient’s body will reabsorb that material so it doesn’t have to be surgically removed. Meanwhile, the clips remain embedded in scar tissue as the reabsorption takes place. So the beacon remains.
Three to six months down the line, the target area can be identified for follow-up treatment, as reliably and accurately as when the marker was first placed. In fact, it can even be seen for post-treatment monitoring, since cancer patients are generally monitored for the rest of their lives.
Recent Research
Two colleagues and I presented the results of research we conducted on the marker at the 2014 American Brachytherapy Society (ABS) annual meeting. In the study, the BioZorb device was implanted in 20 breast cancer patients who were candidates for either partial breast irradiation (PBI) or whole breast irradiation (WBI) boost treatments, which are similar to PBI treatments in that they are focused not on the whole breast, but on the surgical site and the margins where cancer might recur.
Here’s what we found: As the device’s designers intended, the target area was much more clearly delineated with the 3-D marker than it would have been with traditional methods. Because of that, the radiation dose was dramatically decreased for these women. All of them tolerated the device well, and there were no complications. Finally, cosmetic outcomes were excellent.
Comparison With Traditional Methods
This novel device addresses virtually all the shortcomings of traditional markers. To give you a better idea of what a difference this makes, consider what treatment planners and radiation oncologists face when traditional methods are used. Normally the surgical area is identified using one of two methods. In one approach, surgeons randomly place small clips, much like the ones embedded in the 3-D marker, during lumpectomy surgery. Alternatively, treatment planners and radiation oncologists will use a computed tomography (CT) scan to locate external anatomic landmarks and/or tissue changes near the seroma cavity.
There are potential problems with both methods. These older kinds of clips sometimes migrate into the seroma fluid — essentially sinking to the bottom of the pond — and stack up there, making them useless for outlining the surgical cavity. If the surgeon attaches the clips to tissue so they won’t float, they can still migrate away from the cavity as it heals. Even without migration, it’s hard to know what you’re looking at because different surgeons may place the clips at different distances from the surgical site.
As a radiation oncologist whose first priority is to make sure every cancer cell is irradiated, in cases like these I have to target the entire area outlined by the clips and then some, just to account for any possible migration or location issues when the clips were placed.
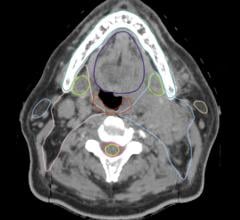
When using a CT scan for targeting, treatment planners and radiation oncologists will look for a fuzzy white dense area they know includes the surgical cavity. But the emphasis is on the word “fuzzy.” Again, because irradiating the patient’s cancer is my first concern, I’m going to target the entire white density out to its fuzzy edges, even though those edges may be beyond the margins that actually need treatment. I have to do that because I can’t always see the margins of the surgical site clearly.
Another challenge involves advances in surgical techniques. Many breast surgeons today use oncoplastic techniques to help reconstruct the breast after lumpectomy surgery. Oncoplastic surgery combines the latest plastic surgery techniques with breast surgical oncological methods to help maintain the breast’s natural shape and symmetry with the other breast. But when this method is used, it may eliminate the seroma cavity — the fuzzy white dense area on the CT scan. That adds to the guesswork for radiation treatment.
Another concern can be the timing of post-surgical chemotherapy. Radiation treatment often does not begin until chemotherapy is completed, which can be as much as five months after surgery. During that period, the surgical cavity may have healed to the point that it can’t be located on a CT scan. In that case radiation needs to be delivered more broadly, which inevitably means more exposure to healthy tissue and structures.
These issues don’t exist with the 3-D marker, as our study confirmed. Even months after surgery, the clips can still be easily seen and their location reliably defines the surgical cavity. Following oncoplastic surgery when this device is used, the surgical cavity can also be accurately located after surgery.
Studies like the one I contributed to are admittedly small. But there have been several such studies in the two years since the 3-D device became available, and the results have been consistently positive. I am confident we are entering a new era of radiation treatment for breast cancer, where the precision of tissue marking is catching up to the precision of radiation delivery. We use the 3-D device regularly in my practice in Arizona for both WBI boost treatment and certain forms of PBI.
It can take a while for new methods to catch on, no matter how beneficial and evidence-based they are. I hope the 3-D device is adopted more quickly than that. Our patients deserve the safest, most precise and most cosmetically satisfying treatment we can provide them. I believe that option is now available for breast cancer patients needing post-surgical radiation treatment.
Robert Kuske, M.D., an internationally known innovator in radiation therapy for breast cancer, is currently a breast cancer-specific radiation oncologist and co-founder of Arizona Breast Cancer Specialists in Scottsdale, Ariz. Previously, he was professor of human oncology at the University of Wisconsin and before that, chief of staff and chairman of radiation oncology at the famed Ochsner Clinic in New Orleans. While in New Orleans, he also helped build and lead the Cancer Center at Tulane Medical School. With colleagues, he pioneered the widely used treatment regimen called accelerated partial breast irradiation (APBI) as an option for select early-stage breast cancer patients.




 April 17, 2024
April 17, 2024