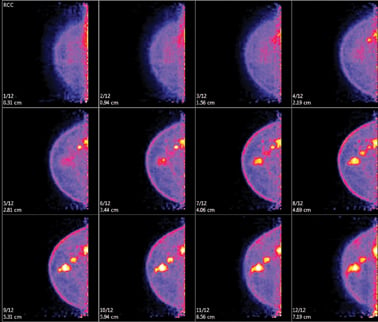
Positron emission mammography (PEM) produces a 3-D tomographic view of the breast in 12 images per view. Photo courtesy of Naviscan.
The last 20 years have brought about great advances in breast cancer detection and treatment. Among them has been the advent of molecular breast imaging tools, based on either gamma technology — breast-specific gamma imaging (BSGI)/molecular breast imaging (MBI) — or high-resolution positron emission tomography (PET) technology — positron emission mammography (PEM). These tools differ from those traditionally used in breast imaging, as they do not evaluate anatomy, as is the case with mammography, ultrasound and magnetic resonance imaging (MRI).
The techniques are similar, in that cancerous cells demonstrate both increased blood flow, as indicated by sestamibi uptake with BSGI/MBI and glucose through F18-fluorodeoxyglucose (FDG) uptake with PEM. Although both exams are currently marketed as “molecular breast imaging,” some have challenged this categorization because sestamibi uptake functions much like MRI, imaging blood flow rather than molecular metabolic activity imaged using PET.
Images for both studies are acquired with the patient seated upright, mimicking standard mammographic views. Interpretation of both exams is much faster than MRI, as fewer images are produced per study. False positives, such as fibroadenomas, are seen on BSGI and PEM, but less frequently than with MRI. PEM can be followed with whole body PET imaging to evaluate for metastatic disease using the same injection of FDG. Both procedures can take up to 40 minutes per exam, and both involve ionizing radiation.
Pros and Cons of BSGI/MBI
BSGI/MBI is breast scintigraphy using either a single- or dual-headed small-field-of-view gamma camera and (99m)Tc-sestamibi (Cardiolite). The technique uses planar single photon emission radionuclide imaging with a cardiac imaging agent and produces a single one-dimensional image per view, similar to mammography. There is increased mitochondrial uptake in cancer cells by sestamibi due to increased vascularity at a certain tumor size which is not impacted by breast density.
The device has been cleared by the U.S. Food and Drug Administration since 1999 and is used mostly as an adjunct to mammography to help differentiate scar tissue from cancer recurrence in patients with prior diagnosis, to screen high-risk patients who are contraindicated for MRI and to help patients with indeterminate findings on mammography. A biopsy accessory for single-headed cameras was cleared by FDA in late 2009, although only a handful of centers performing BSGI have adopted biopsy to date.
Small retrospective studies have shown a sensitivity of 88.5 percent for sub-centimeter cancers and 93 percent for invasive lobular carcinoma, although neither study was statistically significant. Recent prospective trials indicate a sensitivity of 88.8 percent and a specificity of 90 percent for primary lesions ? 1 cm and a sensitivity of 29 percent for lesions smaller than 5 mm.
Dose is a concern with BSGI/MBI, given that the technology is most commonly deployed in a screening population. BSGI requires a dose of 25 mCi of (99m)Tc-sestamibi, and imaging must occur immediately following injection as the radiotracer tends to wash out, especially for aggressive and chemo-resistant cancers.
The published guidelines by the Society of Nuclear Medicine propose a 25 mCi dose of sestamibi, although there are ongoing trials underway to look at dose reduction with dual-headed BSGI/MBI cameras. The currently approved dose results in 830 mrem of radiation exposure to the patient or 1.5 times the average yearly exposure in the United States. Future reduced dose of sestamibi will require off-label use, as the FDA drug labeling currently indicated for breast imaging with this radiotracer is 20-30 mCi. Radiation dose to the technologist is also a concern, given that positioning and imaging of the patient occurs immediately post-injection.
As with breast MRI, BSGI/MBI studies in pre- and peri-menopausal women must be timed appropriately with the patient’s menstrual cycle. An additional challenge is the lack of breast or cancer-specific radiopharmaceuticals for this tool, since sestamibi was developed specifically for cardiac imaging. There do not appear to be any new radiotracers for this type of camera currently available clinically or through research protocols at this time.
Pros and Cons of PEM
PEM, the breast application of a high-resolution PET scanner, has emerged since its FDA clearance in 2003 as a viable advanced imaging technique to help with presurgical planning and staging, to evaluate axillary lymph nodes, to monitor response to neoadjuvant chemotherapy and to look for recurrent disease. A biopsy capability using existing MRI vacuum-assisted techniques was approved in 2008 and has a high adoption rate.
PEM produces a 3-D tomographic view of the breast in 12 images per view. Early studies comparing the results of PEM with pathology showed a sensitivity of 90 percent as well as a high specificity of 86 percent in evaluating known breast cancer or suspicious lesions. Other studies have shown that PEM has a positive predictive value for biopsy of 83 percent when compared to 74 percent for MRI with no statistically significant difference in sensitivity and specificity.
A larger prospective multicenter study showed PEM and MRI to be equally sensitive and specific for detection of primary lesions, but PEM was more specific than MRI for detection of secondary smaller lesions and had a higher PPV. PEM’s higher specificity shows a potential to reduce false positives compared to other advanced breast imaging modalities.
PEM requires a high-resolution PET scanner and injection of 5 mCi of 18F-FDG, a minimum of 60-minute uptake time with a fasting glucose level of <140 mg/dl. No specific timing is required, as the radiotracer is not impacted by hormonal perturbations. It should be performed by a dedicated team, which can include either radiologists experienced in mammography and image-guided biopsy techniques or nuclear medicine physicians. The availability of PEM-guided vacuum-assisted biopsy is essential for centers performing PEM to confirm lesions not seen on any other modality, given the scanner’s 1.6 mm resolution.
As this technology is PET, new breast cancer-specific radiotracers, such as fluorine-18 16?-fluoroestradiol (FES), and tracers developed to identify proliferating and non-proliferating tumors, like [18F] fluorothymidine (18F -FLT) and Cu-64, are currently used on this device to monitor response to neoadjuvant chemotherapy. These radiotracers are available only in clinical trials under IND in the U.S., but are in clinical use with PEM in other countries.
Dose is also a concern with PEM. Since it is typically deployed after an initial diagnosis of cancer, the risk-to-benefit ratio should be adjusted accordingly. Recent studies have validated a reduced dose of 5 mCi of FDG per exam, resulting in 350 mrem of radiation exposure to the patient or about half the average U.S. yearly exposure. Dose to the technologist with PEM is lower than with BSGI/MBI, as patients are imaged post-void, one hour after injection.
In the United States, PEM is not currently deployed as a screening tool and may only be used post-diagnosis. BSGI/MBI is often used both pre- and post-diagnosed, and many centers have taken advantage of Henda’s Law to deploy the technology for problem-solving equivocal mammograms in dense-breasted patients. These studies are only reimbursed if BI-RADS 0 is given on a mammography report.
Conclusion
Although breast MRI is well-suited to image patients at high risk for breast cancer, it is estimated that 15 percent of women cannot tolerate this procedure due to severe claustrophobia, body habitus, kyphosis or other contradindications. For this reason, BSGI or PEM may help guide breast evaluation in selected patient populations.
As with breast MRI, findings from these molecular imaging tools should not be substituted for histological tissue diagnosis, especially when the patient and her surgeon are considering breast conservation. The decision to use these tools as an adjunct to the evaluation of patients with breast cancer should be made by the physician and the patient after joint consideration of the benefits as well as the risks associated with exposure to radiation. Neither BSGI/MBI nor PEM should replace mammography for yearly screening exam in the general population, given the cost to the healthcare system and the radiation exposure.
Parvez Shirazi, M.D., is founder of Golf Diagnostic Imaging Center, a multi-modality imaging center located in the Chicago area. He is also professor of nuclear technology at King Edward Medical College in Lahore, Pakistan, associate professor at Loyola University Chicago and is adjunct faculty at the University of St. Francis in Joliet, Ill.
References:
1. Kim BS. Usefulness of breast-specific gamma imaging as an adjunct modality in breast cancer patients with dense breast: a comparative study with MRI. Ann Nucl Med. 2011 Oct 18.
2. O’Connor MK, Phillips SW, Hruska CB, Rhodes DA. Molecular breast imaging: advantages and limitations of a scintimammographic technique in patients with small breast tumors. Breast J. 2007 Jan-Feb;13(1):3-11.
3. Brem RF, Floerke AC, Rapelyea JA, Teal C, Kelly T, Mathur V. Breast-specific gamma imaging as an adjunct imaging modality for the diagnosis of breast cancer. Radiology. 2008 Jun;247(3):651-7.
4. Tadwalkar RV, Rapelyea JA, Torrente J, Rechtman LR, Teal CB, McSwain AP, Donnelly C, Kidwell AB, Coffey CM, Brem RF. Breast-specific gamma imaging as an adjunct modality for the diagnosis of invasive breast cancer with correlation to tumour size and grade. Br J Radiol. 2011 Jun 28.
5. Brem RF, Ioffe M, Rapelyea JA, Yost KG, Weigert JM, Bertrand ML, Stern LH. Invasive lobular carcinoma: detection with mammography, sonography, MRI, and breast-specific gamma imaging. AJR Am J Roentgenol. 2009 Feb;192(2):379-83.
6. Berg WA, Weinberg IN, Narayanan D, Lobrano ME, Ross E, Amodei L, Tafra L, Adler LP, Uddo J, Stein W 3rd, Levine EA; Positron Emission Mammography Working Group. High-resolution fluorodeoxyglucose positron emission tomography with compression (“positron emission mammography”) is highly accurate in depicting primary breast cancer. Breast J. 2006 Jul-Aug;12(4):309-23.
7. Berg WA, Madsen KS, Schilling K, Tartar M, Pisano ED, Larsen LH, Narayanan D, Ozonoff A, Miller JP, Kalinyak JE. Breast cancer: comparative effectiveness of positron emission mammography and MR imaging in presurgical planning for the ipsilateral breast. Radiology. 2011 Jan;258(1):59-72. Epub 2010 Nov 12.
8. Schilling K, Narayanan D, Kalinyak JE, The J, Velasquez MV, Kahn S, Saady M, Mahal R, Chrystal L. Positron emission mammography in breast cancer presurgical planning: comparisons with magnetic resonance imaging. Eur J Nucl Med Mol Imaging. 2011 Jan;38(1):23-36. Epub 2010 Sep 25.
9. Berg WA, Blume JD, Adams AM, Jong RA, Barr RG, Lehrer DE, Pisano ED, Evans WP 3rd, Mahoney MC, Hovanessian Larsen L, Gabrielli GJ, Mendelson EB. Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666. Radiology. 2010 Jan;254(1):79-87.
10. Kalinyak JE, Schilling K, Berg WA, Narayanan D, Mayberry JP, Rai R, Dupree EB, Shusterman DK, Gittleman MA, Luo W, Matthews CG. PET-guided breast biopsy. Breast J. 2011 Mar-Apr;17(2):143-51.
11. Tafra L. Positron Emission Tomography (PET) and Mammography (PEM) for breast cancer: importance to surgeons. Ann Surg Oncol. 2007 Jan;14(1):3-13. Review
12. Tafra L, Cheng Z, Uddo J, Lobrano MB, Stein W, Berg WA, Levine E, Weinberg IN, Narayanan D, Ross E, Beylin D, Yarnall S, Keen R, Sawyer K, Van Geffen J, Freimanis RL, Staab E, Adler LP, Lovelace J, Shen P, Stewart J, Dolinsky S. Pilot clinical trial of 18F-fluorodeoxyglucose positron-emission mammography in the surgical management of breast cancer. Am J Surg. 2005 Oct;190(4):628-32.
13. Narayanan D, Madsen KS, Kalinyak JE, Berg WA. Interpretation of positron emission mammography and MRI by experienced breast imaging radiologists: performance and observer reproducibility. AJR Am J Roentgenol. 2011 Apr;196(4):971-81.
14. Keto JL, Kirstein L, Sanchez DP, Fulop T, McPartland L, Cohen I, Boolbol SK. MRI versus breast-specific gamma imaging (BSGI) in newly diagnosed ductal cell carcinoma-in-situ: a prospective head-to-head trial. Ann Surg Oncol. 2012 Jan;19(1):249-52. Epub 2011 Jul 8.
15. Wahner-Roedler DL, Boughey JC, Hruska CB, Chen B, Rhodes DJ, Tortorelli CL, Maxwell RW, Cha SS, O’Connor MK. The use of molecular breast imaging to assess response in women undergoing neoadjuvant therapy for breast cancer: a pilot study. Clin Nucl Med. 2012 Apr;37(4):344-50.
16. Goldsmith SJ, Parsons W, Guiberteau MJ, Stern LH, Lanzkowsky L, Weigert J, Heston TF, Jones E, Buscombe J, Stabin MG; Society of Nuclear Medicine. SNM practice guideline for breast scintigraphy with breast-specific gamma-cameras J Nucl Med Technol. 2010 Dec;38(4):219-24. Epub 2010 Nov 5.
17. FDA label for CARDIOLITE Kit for the Preparation of Technetium Tc99m Sestamibi for Injection: www.accessdata.fda.gov/drugsatfda_docs/label/2008/019785s018lbl.pdf
18. FDA label for FDG F 18 Injection: www.accessdata.fda.gov/drugsatfda_docs/label/2005/021870lbl.pdf
19. Moadel RM. Breast cancer imaging devices. Semin Nucl Med. 2011 May;41(3):229-41. Review.
20. Schilling, K. “Radiotracer Dose Reduction in High Resolution Breast PET Imaging.” NCoBC – 22nd Annual National Interdisciplinary Meeting Breast Center Conference, March, 2012, Las Vegas, NV
21. Mankoff, D. “FDG-PET in Assessing Tumor Response.” Breast Cancer Imaging: State of the Art 2011, April 22, 2011, NIH, Bethesda, MD
22. Texas HB 2102 Henda’s Law: www.txrad.org/index.cfm/trs-forum/texas-hb-2102/



 April 15, 2024
April 15, 2024