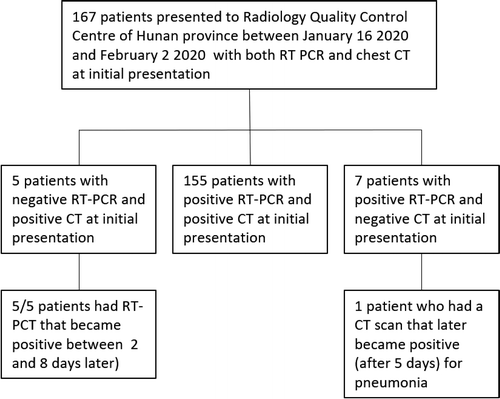
Figure 1: Patient flowchart. Of 167 patients screened, 5 (3%) had negative RT-PCR results and chest CT findings compatible with 2019-nCoV pneumonia. Chart courtesy of Radiology
As the 2019-nCoV Pneumonia is taking the world by storm, researchers have found a possible way to predict this virus through computed tomography (CT) evidence. Their reportings, “Chest CT for Typical 2019-nCoV Pneumonia: Relationship to Negative RT-PCR Testing,” led by Xingzhi Xie from Central South University in Hunan Province, was published Feb. 12 in an online version of Radiology. The study showed CT imaging was able to identify coronavirus before some patients tested positive in laboratory tests.
The detection of novel coronavirus has largely been done using reverse transcription polymerase chain reaction (RT-PCR), This laboratory technique combines reverse transcription of RNA into DNA and amplification of specific DNA targets to identify DNA that marches that of the virus.
The purpose of the new radiology study was to describe CT imaging features of five patients who initially tested negative for coronavirus in RT-PCR tests, or had weak positive results, but were highly suspected of infection.
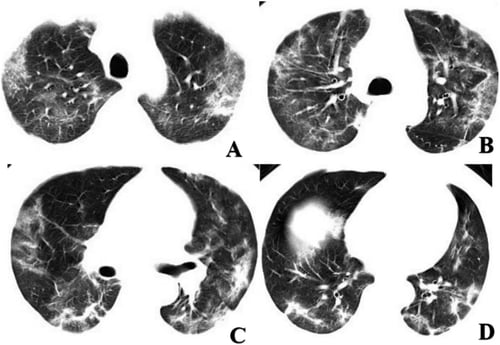
Chest CT imaging of a coronavirus patient showing multifocal GGO and mixed consolidation that most appeared at peripheral area of both lungs. The CT involvement score was 11. Image courtesy of Radiology
The study evaluated 167 patients; of these 5 (3 percent) initially had negative RT-PCR, but had positive chest CT with a pattern consistent with viral pneumonia. After positive CT findings, all patients were isolated for presumed 2019-nCoV pneumonia. Repeat swab testing and RT-PCR tests 2019-nCoV infection in all patients. In 7 patients (4 percent), CT was initially negative, while RT-PCR was positive for novel coronavirus. In 155 patients (93 percent), both RT-PCR and CT were concordant for 2019-nCoV infection. Of the 5 patients with negative RT-PCR and positive CT at initial presentation, the median CT involvement score was 4. The highest CT involvement score was 14 while the minimum was 2. Figure 1 illustrates a patient flowchart.
The authors commented that while a swab test is the standard assessment for 2019-nCoV infection diagnosis, current tests are time-consuming and scarce due to the high demand. In addition, RT-PCR testing for coronavirus may be falsely negative due to laboratory error or insufficient viral material in the specimen.
The study authors concluded: “Previous radiographic studies showed that the majority of cases had similar features on CT images, like ground glass opacities (GGO) or mixed GGO and consolidation. 2019-nCoV pneumonia is likely to have a peripheral distribution with bilateral, multifocal lower lung involvement. In the context of typical clinical presentation and exposure to other individuals with 2019-nCoV, CT features of viral pneumonia may be strongly suspicious for 2019-nCoV infection despite negative RT-PCR results. In these cases, repeat swab testing and patient isolation should be considered.”
Additional Novel Coronavirus Chest CT Studies
Radiology published another study, “Time Course of Lung Changes on Chest CT During Recovery from 2019 Novel Coronavirus (COVID-19) Pneumonia,” Feb. 13, 2020.
The purpose of this study was to determine the change in chest CT findings associated with COVID-19 pneumonia from initial diagnosis until patient recovery. We studied patients with COVID-19 at approximately 4-day intervals from the onset of disease until recovery. Patients with complicated pneumonia including severe respiratory distress (respiratory rate >30 breaths/min), requirement for oxygen treatment or mechanical ventilation or SpO2 lower than 90 percent on room air were excluded. In patients without severe respiratory disease, the major pulmonary CT findings of novel coronavirus pneumonia were GGO, crazy-paving pattern and consolidation predominantly in subpleural locations in the lower lobes. Chest CT abnormalities increased in number and severity of lesions in the first 10 weeks (peaking at approximately 10 days). Subsequently, there was a short plateau phase and a gradual decrease in abnormalities. You can view full results here.
In this study, the patients underwent multiple pulmonary CT scans (≥3 times) providing reliable data of the dynamic radiological pattern. The typical mild COVID-19 pneumonia mainly starts as small subpleural, unilateral or bilateral GGO in the lower lobes, which then develops into the crazy-paving pattern and subsequent consolidation. After more than two weeks, the lesions are gradually absorbed with residual GGO and subpleural parenchymal bands. In these patients who recovered from COVID- 19 pneumonia, 4 stages of lung involvement were defined on CT:
1. Early stage (0-4 days after onset of the initial symptom): In this stage, GGO was the main radiological demonstration (Figure 2a) distributed subpleurally in the lower lobes unilaterally or bilaterally. In our ochort, negative findings were revealed in four patients (total CT score=0) (Figure 3a). However, repeat pulmonary CT showed lung abnormalities in these 4 patients.
2. Progressive stage (5-8 days after the onset of the initial symptom): In this stage, the infection rapidly aggravated and extended to a bilateral multi-lobe distribution with diffuse GGO, crazy-paving pattern and consolidation (Figure 5b).
3. Peak stage (9-13 days after the onset of the initial symptom): In this stage, the involved area of the lungs slowly increased to the peak invovlment and dense consolidation became more prevalent. Findings included diffuse GGO, crazy-paving pattern, consolidation, and residual parenchymal bands.
4. Absorption stage (≥14 days after the onset of the initial symptom): In this stage, the infection was controlled and the consolidation was gradually absorbed. No crazy-paving pattern was present anymore. However, in this process, extensive GGO could be observed as the demonstration of the consolidation absorption (Figure 5d). Noticeably, in this study, no crazy-paving was observed in this stage, likely as a result of recovering. Based on the total CT score, the absorption stage extended beyond 26 days (our last days of follow-up) from the onset of initial symptoms.
FDA Efforts to Prepare to Combat 2019-nCoV
In a significant step toward the coronavirus efforts, on Feb. 13, the U.S. Food and Drug Administration (FDA) reached a major milestone by issuing an emergency use authorization (EUA) for the first 2019 Novel Coronavirus diagnostic. This authorization enables emergency use of the Centers for Disease Control and Prevention’s (CDC) 2019-nCoV Real-Time RT-PCR Diagnostic Panel. To date, this test has been limited to use at CDC laboratories. This authorization allows the use of the test at any CDC-qualified lab across the country
“Since this outbreak first emerged, we’ve been working closely with our partners across the U.S government and around the globe to expedite the development and availability of critical medical products to help end this outbreak as quickly as possible. This continues to be an evolving situation and the ability to distribute this diagnostic test to qualified labs is a critical step forward in protecting the public health,” said FDA Commissioner Stephen M. Hahn, M.D. “Our collaboration with the CDC has been vital to rapidly developing and facilitating access to this diagnostic test. The FDA remains deeply committed to utilizing our regulatory tools and leveraging our technical and scientific expertise to advance the availability of critical medical products to respond to this outbreak in the most expeditious, safe and effective manner possible.”
The 2019-novel coronavirus (2019-nCoV), identified in Wuhan, China in December 2019, is a new type of coronavirus that can cause severe respiratory illness in humans. Most patients with confirmed 2019-nCoV infection have developed fever and/or symptoms of acute respiratory illness (e.g., cough, difficulty breathing). However, limited information is currently available to characterize the full spectrum of clinical illness associated with 2019-nCoV infection. To date most reported cases of 2019-nCoV infection outside of China have been linked to residence in or travel to Wuhan, China. At this time, federal health officials continue to believe that the threat to the general American population from this virus is relatively low.
Under this EUA, the use of 2019-nCoV Real-Time RT-PCR Diagnostic Panel is authorized for patients who meet the CDC criteria for 2019-nCoV testing. Testing is limited to qualified laboratories designated by the CDC and, in the U.S., those certified to perform high complexity tests. The diagnostic is a reverse transcriptase polymerase chain reaction (PCR) test that provides presumptive detection of 2019-nCoV from respiratory secretions, such as nasal or oral swabs. A positive test result indicates likely infection with 2019-nCoV and infected patients should work with their health care provider to manage their symptoms and determine how to best protect the people around them. Negative results do not preclude 2019-nCoV infection and should not be used as the sole basis for treatment or other patient management decisions. Negative results must be combined with clinical observations, patient history and epidemiological information.
The FDA can issue an EUA to permit the use, based on scientific data, of certain medical products that may be effective in diagnosing, treating or preventing such disease or condition when there is a determination, by the Secretary of Health and Human Services (HHS), that there is a public health emergency or a significant potential for a public health emergency that has a significant potential to affect national security or the health and security of U.S. citizens, and a declaration that circumstances exist justifying the medical products’ emergency use.
On Jan. 31, HHS Secretary Alex Azar declared a public health emergency recognizing the potential threat that 2019-nCoV poses and reiterating the government’s dedication to leveraging all available resources to help prevent, mitigate and respond to this threat. As there are no commercially available diagnostic tests cleared or approved by the FDA for the detection of 2019-nCoV it was determined that an EUA is crucial to ensure timely access to diagnostics. The HHS Secretary accordingly made the necessary EUA determination and declaration and the FDA issued this EUA in response to a request from the CDC. This action is the result of the close collaboration between the FDA, the CDC and the Centers for Medicare and Medicaid Services, which provides oversight for U.S. laboratories, to prioritize the efficient development and implementation of critical medical products in response to emerging infectious disease outbreaks, such as novel coronavirus.
The FDA outlined its approach to expediting the development and availability of critical medical products to prevent, diagnose and treat 2019-nCoV using all applicable regulatory authorities to respond to this outbreak on Jan. 27. The agency remains committed to working with developers, international partners and the U.S. government to help support this public health response. The FDA is dedicated to actively working with other 2019-nCoV diagnostic developers to help accelerate development programs and requests for EUAs, in fact several have already requested and received the EUA template for this outbreak. The FDA, among other steps, is providing its highest level of attention to helping expedite the development and review of a variety of medical products being developed to diagnose, treat and prevent the spread of this outbreak.
CMS Develops New Codes for Coronavirus Testing
In addition to the FDA’s efforts, the Centers for Medicare and Medicaid Services (CMS) took further action to ensure America’s healthcare facilities and clinical laboratories are prepared to respond to the threat of the 2019-Novel Coronavirus (COVID-19). Specifically, CMS developed a new Healthcare Common Procedure Coding System (HCPCS) code for providers and laboratories to test patients for SARS-CoV-2. This code will allow those labs conducting the tests to bill for the specific test instead of using an unspecified code, which means better tracking of the public health response for this particular strain of the coronavirus to help protect people from the spread of this infectious disease.
Healthcare providers who need to test patients for Coronavirus using the CDC 2019 Novel Coronavirus Real Time RT-PCR Diagnostic Test Panel may bill for that test using the newly created HCPCS code (U0001). The Medicare claims processing system will be able to accept this code on April 1, 2020 for dates of service on or after Feb. 4, 2020. HCPCS is a standardized coding system that Medicare and other health insurers use to submit claims for services provided to patients.
Following is a summary of CMS Public Health Action on Coronavirus to date:
- On February 6, 2020, CMS issued a memo to help the nation’s healthcare facilities take critical steps to prepare for COVID-19. To view a copy of the memo and see more details: https://www.cms.gov/medicareprovider-enrollment-and-certificationsurveycertificationgeninfopolicy-and-memos-states-and/information-healthcare-facilities-concerning-2019-novel-coronavirus-illness-2019-ncov
- On February 6, 2020, CMS also gave CLIA-certified laboratories information about how they can test for SARS-CoV-2. To read more about those efforts: https://www.cms.gov/medicareprovider-enrollment-and-certificationsurveycertificationgeninfopolicy-and-memos-states-and/notification-surveyors-authorization-emergency-use-cdc-2019-novel-coronavirus-2019-ncov-real-time-rt
Additional References on the Novel Coronavirus:
1. Chest CT for Typical 2019-nCoV Pneumonia: Relationship to Negative RT-PCR Testing: Radiology. https://doi.org/10.1148/radiol.2020200343. Accessed Feb. 14, 2020
2. Time Course of Lung Changes On Chest CT During Recovery From 2019 Novel Coronavirus (COVID-19) Pneumonia: Radiology. https://doi.org/10.1148/radiol.2020200370. Accessed Feb. 14, 2020
3. U.S. Food and Drug Administration: https://www.fda.gov/news-events/press-announcements/fda-takes-significant-step-coronavirus-response-efforts-issues-emergency-use-authorization-first?utm_campaign=020420_PR_FDA%20Issues%20EUA%20for%20First%202019%20Novel%20Coronavirus%20Diagnostic&utm_medium=email&utm_source=Eloqua. Accessed Feb. 14, 2020.
4. Public Health News Alert: CMS Develops New Code for Coronavirus Lab Test. https://www.cms.gov/. Accessed Feb. 14, 2020.
Related Coronavirus Imaging Content:
Radiologists Describe Coronavirus CT Imaging Features
CT Imaging of the 2019 Novel Coronavirus (2019-nCoV) Pneumonia
Infervision in the Frontlines Against the Coronavirus
CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV)
Chest CT Findings of Patients Infected With Novel Coronavirus 2019-nCoV Pneumonia










 April 17, 2024
April 17, 2024