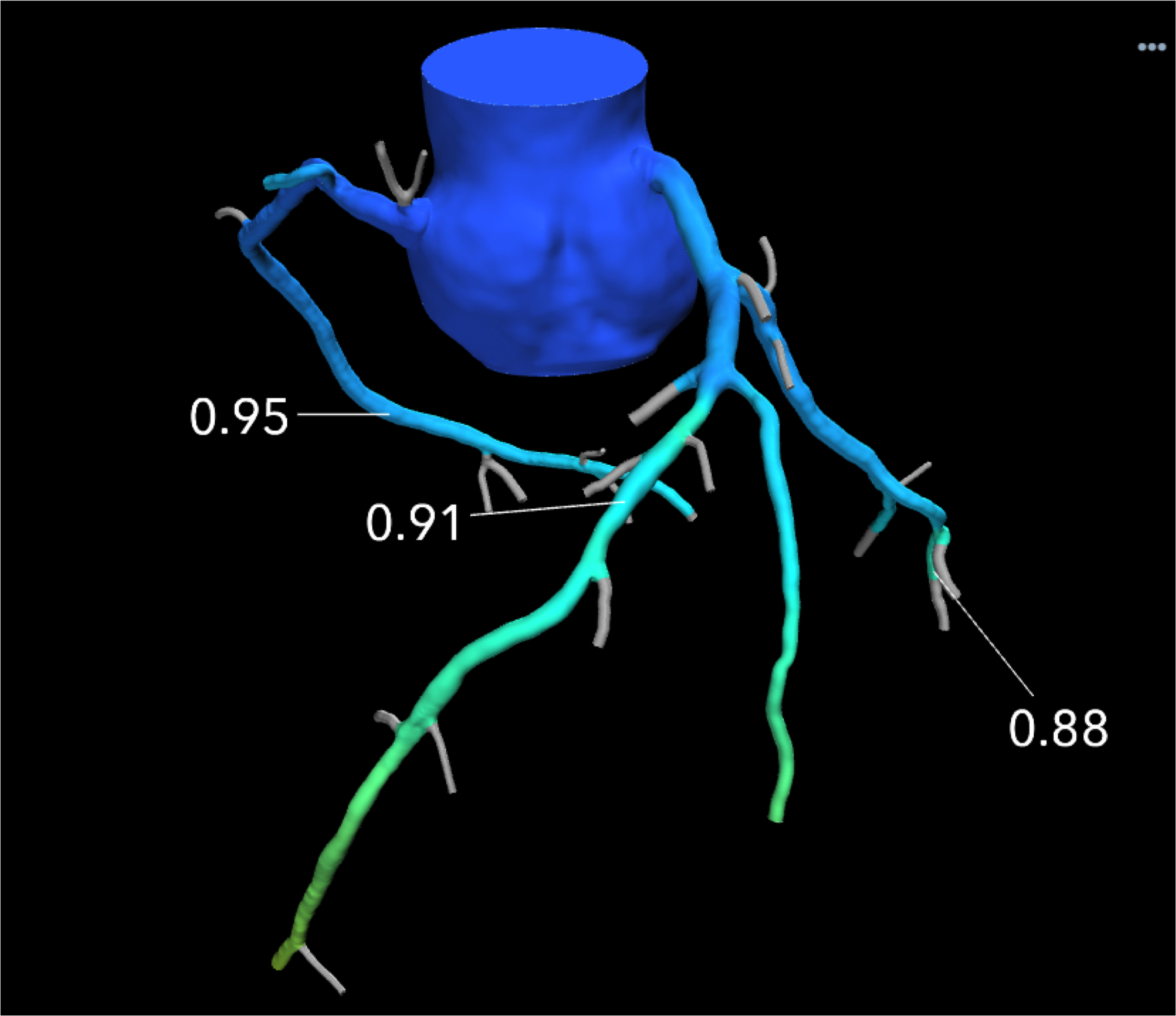
An example of a HeartFlow FFR-CT image showing the blood flow through what looked like a significant blockage on CT angiography alone, actually was not flow-limiting based on computational fluid dynamics. Use of the technology was supposed to reduce the number of diagnostic catheterizations in the FORECAST trial, but the costs of FFR-CT were not offset enough to show cost savings.
October 22, 2020 – In the FORECAST randomized clinical trial, the use of fractional flow reserve (FFR) derived from computed tomography (FFR-CT) did not significantly reduce costs, but did reduce the use of invasive coronary angiography (ICA). This study was looking to confirm that FFR-CT use would result in a lowering of costs to care for patients in the U.K. National Health System (NHS).
Findings were reported at the 2020 Transcatheter Cardiovascular Therapeutics (TCT) Connect virtual meeting.
"In patients presenting with new onset stable chest pain, a strategy of CT coronary angiography with FFR-CT, when compared with a strategy of routine care, it did not significantly reduce costs in the NHS system. But, it was associated with a significantly lower rate of invasive angiography," explained Nick Curzen, BM (Hons), Ph.D., chair of interventional cardiology and professor of interventional cardiology, University of Southampton, United Kingdom.
FFR-CT is a novel, validated, non-invasive method for describing both the amount of coronary atheroma from a CT coronary angiogram (CTCA), but also vessel-specific ischemia derived from the CTCA and other clinical parameters using a fluid dynamics computer model. Previous studies have indicated that FFR-CT reduces the uptake of invasive angiography that shows no significant coronary artery disease (CAD), without compromising patient safety. The clinical effectiveness and economic impact of using FFR-CT instead of other tests in the evaluation of patients with stable chest pain has not yet been tested in a randomized trial, although based upon cost models on observational data, FFR-CT is already recommended in routine clinical practice by National Institute for Health and Care Excellence (NICE) in the U.K., because it appeared cost dominant.
“Results from FORECAST indicate that CTCA and FFR-CT as a frontline strategy may not be associated with the financial savings projected from observational data by NICE,” said Curzen. “However, the reduction in invasive coronary angiography is important and will be very attractive to patients. More data is needed to determine the optimal use for FFR-CT in clinical practice.”
"I am wondering if you missed the endpoint of the cost savings because in the U.K. it seems 66 percent of the reference strategy was already using CTA. Is it just that it is too late now to show there is a cost savings because CTA is already used as a front-line strategy?" asked Roxana Mehran, M.D. She is The Mount Sinai professor of clinical research and outcomes, professor of medicine (cardiology), population health science and policy, Icahn School of Medicine at Mount Sinai and director of interventional cardiovascular research and clinical trials, The Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai.
Curzen said this is good question, since the NICE guidelines changed just prior to the start of this trial to support use of CTA. However, he said the trial was not set up to compare CTA vs. invasive angiography, it was set up to look at costs alone.
"The fact is, the invasive coronary angiography rate and the revascularization rate were not reduced enough to take account of the costs for the CCTA and FFR-CT up front. The trial question is if FFR-CT can save money. I think we can reach that point, but not by using it so freely," Curzen said.
"In order for a test to lower costs, it is going to have to provided fairly substantial resource offsets, and in this study it simply did not," explained David Cohen, M.D., chair in cardiovascular clinical research at Saint Luke’s Mid America Heart Institute. "The only way FFR-CT is going to lead to cost saving is if it can offset other tests, like avoiding the need for nuclear stress tests."
Clinical Results and Financial Impact of Using FFR-CT
The primary endpoint of the FORECAST trial was resource utilization derived from non-invasive cardiac tests, invasive angiography, coronary revascularization, hospitalization for a cardiac event, and cardiac medications at nine months. Prespecified secondary endpoints included major adverse cardiac and cerebrovascular events, revascularization, angina severity, and quality of life (QOL).
In the trial, 1,400 patients with stable chest pain at 11 U.K. centers were randomized to receive either CCTA with FFR-CT of lesions with stenosis severity of 40% or greater (test arm, n=699) or routine assessment as directed by the NICE Guideline for Chest Pain of Recent Onset (reference arm, n=700). The routine assessment arm included a mixture of non-invasive tests, including CCTA (without FFR-CT) in 61.4% of subjects. The mean age of the overall population was 60 (25-89) years and 52% were male. Baseline demographics, angina status, and QOL/health status were similar between the groups.
In patients presenting with new onset stable chest pain, a strategy of CTCA with FFR-CT, when compared with a strategy of routine care, did not significantly reduce average total costs in the NHS system (£1,605.50 vs. £1,491.46, p=0.962). At nine months, the number of patients in the test arm who underwent the following non-invasive tests were: CTCA (674), FFRCT (220), stress echo (13), perfusion scan (4), stress MRI (15), exercise ECG (27). The number of patients in the reference arm who underwent these tests were: CTCA (460), FFRCT (9), stress echo (124), perfusion scan (34), stress MRI (20), and exercise ECG (99). A total of 22% fewer patients in the test group had invasive coronary angiography (ICA) compared to the reference group (136 vs. 175, p=0.01). There was no significant difference in the rates of MACCE or revascularization.
The FORECAST trial was an investigator-initiated study with an unrestricted research grant from HeartFlow. Dr. Curzen reported the following disclosures: speaker fees and travel sponsorship from HeartFlow in the last 3 years.
Find additional TCT 2020 news, video and late-breaking studies
Related FFR-CT Content:
VIDEO: Use of FFR-CT to Non-invasively Evaluate Coronary Lesion Severity — Interview with James Udelson, M.D.
Novel FFR Methods Can Reduce Procedure Time and Cost
Current Evidence for Cardiac CT Calls for Change in Recommendations and Reimbursements
New Technologies Take Cardiac CT to the Next Level
Image-based FFR May Replace Pressure Wires and Adenosine
New Technology Directions in Fractional Flow Reserve (FFR)
8 Cardiovascular Technologies to Watch in 2020
VIDEO: Using FFR-CT in Everyday Practice
FFR-CT is Ready for Prime-time Evaluation of Coronary Disease


 April 12, 2024
April 12, 2024