October 21, 2016 — The U.S. Food and Drug Administration (FDA) issued a letter calling on radiation oncologists, medical physicists, dosimetrists and radiation therapists to stop using all medical devices manufactured and sold by Multidata Systems International Corp. The FDA said it is concerned about the risks to patients from the use of devices, which never received FDA market clearance.
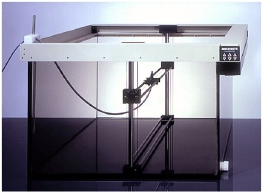
William Maisel, M.D., MPH, deputy for the Center Director for Science, Center for Devices and Radiological Health, FDA, said the agency knows of at least two Multidata medical devices that the company manufactured and distributed in the United States for which FDA never received nor reviewed 510(k) premarket notifications. These devices include: (1) accessories to radiation therapy devices including the Real Time Dosimetry (RTD) Waterphantom System; and (2) the Dual Channel Electrometer. In addition, Multidata has not registered or listed their devices with the FDA, as required by federal law.
Since 2003, Multidata has been under a Consent Decree of Permanent Injunction entered by the U.S. District Court for the Eastern District of Missouri (civil action no. 4:03cv575). Maisel said the decree prohibits Multidata from designing, manufacturing, processing, and distributing medical devices, among other restrictions. The FDA has learned, however, that Multidata manufactured and distributed medical devices in violation of the decree, including repairing and exchanging waterphantom devices for newer design models.
“The FDA is concerned that healthcare providers may be unaware of the risks associated with these devices and urges facilities to discontinue the use of any devices manufactured by Multidata,” Maisel said. He explained these need to be dispose of appropriately and replaced with accessories to radiation therapy devices and radiation treatment planning software that have been reviewed and cleared by FDA.
Analysis of the Problem
At this time, it is not known how many devices manufactured by Multidata are currently in use in hospitals and clinics throughout the United States. The FDA is not aware of adverse event reports associated with the use of Multidata's devices since Multidata has been under the decree, but because the FDA has not received or reviewed 510(k) premarket notifications, the agency is concerned about the potential risk to patients from the devices.
FDA Recommendations
In a letter to providers, Maisel urged discontinuing the use of any devices manufactured by Multidata and disposal of them appropriately. The FDA urges use of accessories to radiation therapy devices and radiation treatment planning software that have been cleared by the FDA. Registered manufacturers of these types of devices are listed under the IYE product code in the FDA’s Registration and Listing Database (www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRL/rl.cfm).
Maisel also said centers should follow quality assurance procedures to verify treatment plans by independent means, which may include manual calculations or measurements of radiation doses.
FDA Actions
Through inspections, the FDA learned that Multidata was designing, manufacturing, processing and distributing medical devices in violation of the decree. As a result, on March 3, 2016, the FDA sent a letter to Multidata ordering the firm to cease designing, manufacturing, processing, packing, repacking, labeling, installing, holding for sale and distributing any medical device. The company has now permanently ceased operations and will be dissolving.
The FDA will keep the public informed if significant new information becomes available, Maisel said.


 April 12, 2024
April 12, 2024