Photo courtesy of GE Healthcare
Computed tomography (CT) continues to be a workhorse modality for radiology departments and its utility keeps expanding. Below are just a few of the ways CT applications have expanded in the last 12 months.
Lung CT
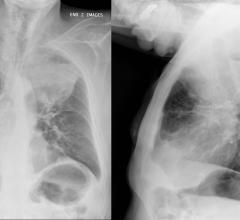
One of the newer applications to develop for CT imaging is as a screening tool for lung cancer and other lung diseases. The application got a major boost when the U.S. Preventive Services Task Force (USPSTF) gave its approval in 2013 for annual lung CT scans for current and former smokers ages 55-80 with a 30-pack year smoking history. The American Cancer Society (ACS) also gave its approval for low-dose computed tomography (LDCT) lung cancer screening in 2013, recommending it, but reducing the upper age limit slightly to 74.
One reason that lung CT screening is increasing is that the dose required continues to go down. In July, Samsung introduced the BodyTom Elite, an updated version of its 32-slice BodyTom system that, among other applications, can perform lung CT exams under 3 mGy.
Several groups in the last 12 months have looked at applying artificial intelligence (AI) algorithms to lung CT to aid radiologists in detection of nodules or masses. In one example, Fujitsu Laboratories Ltd. of Japan shared initial results from an AI algorithm designed to retrieve similar, disease case images from a CT database of previous exams. This particular deep learning algorithm focuses on more diffuse lung diseases like pneumonia, where abnormal shadows spread through the lung in three dimensions, making identification more time-consuming. The algorithm managed 85 percent accuracy for the first five images drawn for each case.
While lung CT screening has proven its merits, there are still various populations who are unable to take advantage of it. Samsung Neurologica and the Levine Cancer Institute of the Carolinas Healthcare System worked together to launch the nation’s first mobile lung CT unit in March. The cancer center put a BodyTom CT unit inside a truck to bring lung cancer diagnosis and intervention to underserved populations across the Carolinas; the goal of the project is to eliminate common barriers to screening such as finances, drive time and lack of public transportation.
Cardiac CT
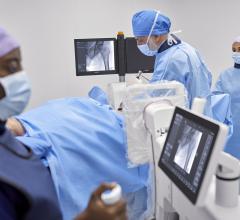
This year saw the introduction of the first dedicated cardiac CT scanner at the American College of Cardiology (ACC) Scientific Session and Expo in March. Developed by GE Healthcare in partnership with Israeli startup Arineta, the Cardiographe is a 64-slice scanner with 140 mm of anatomical coverage, allowing a scan of the entire heart in one rotation without stitching. A gantry speed of 0.24 seconds freezes cardiac motion to eliminate blurring and improve image quality. Other advanced features include dose reduction, iterative reconstruction and metal artifact reduction.
Spatial resolution will continue to be a moving target for CT manufacturers as they attempt to improve viewing of smaller vessels. At the 2017 Society of Cardiovascular Computed Tomography (SCCT) annual scientific meeting, Toshiba offered glimpses of a prototype scanner with a spatial resolution of 0.25 mm. The upper limit for most scanners is 0.50 mm, at which range stents can be seen inside the vessel, but the image is often blurry. With the 0.25 spatial resolution, viewers may be able to see which vendor’s stent was used, as well as individual stent struts and any broken struts.
FFR-CT
One of the most common cardiac applications for CT has been employing CT angiography (CTA) to diagnose patients who present with chest pain, a very unspecific symptom. While CTA does provide a noninvasive method for assessing the coronary arteries, it is less helpful in determining which lesion(s) may be problematic and require intervention. The majority of patients are sent on to the cath lab for an invasive angiogram and potentially catheter-based fractional flow reserve (FFR) measurements. In 2014, the U.S. Food and Drug Administration (FDA) approved a new technology that combines CT with virtual FFR assessment to provide both anatomical and functional assessment of the coronary arteries. FFR-CT technology has been validated in clinical trials to date, reducing the number of patients sent to the cath lab by nearly two-thirds and in turn cutting healthcare costs 33 percent.
At present, HeartFlow Inc. has released the only commercially available version of FFR-CT technology, which requires users to send scans to HeartFlow to perform the calculations and send the studies back to the hospital. This has resulted in relatively slow turnaround times, but the company is working to bring those times down.
Other companies such as Toshiba have their own FFR-CT technology in development, but in the meantime other vendors are partnering with HeartFlow to expand the technology’s reach. Most recently, Philips announced a collaboration agreement with HeartFlow to promote the latter’s analysis in conjunction with Philips’ advanced catheters for coronary FFR, instant wave-free ratio (iFR) and intravascular ultrasound (IVUS) solutions. The two companies will also work together to develop an improved cath lab X-ray angiography image-derived FFR or iFR solution, with the goal of enhancing workflow in the cath lab and improving diagnosis of coronary artery disease (CAD). Siemens Healthineers and GE also announced partnerships with HeartFlow in 2017.
Read the related 2018 article "New Cardiovascular CT Technology Entering the Market."


 April 24, 2024
April 24, 2024