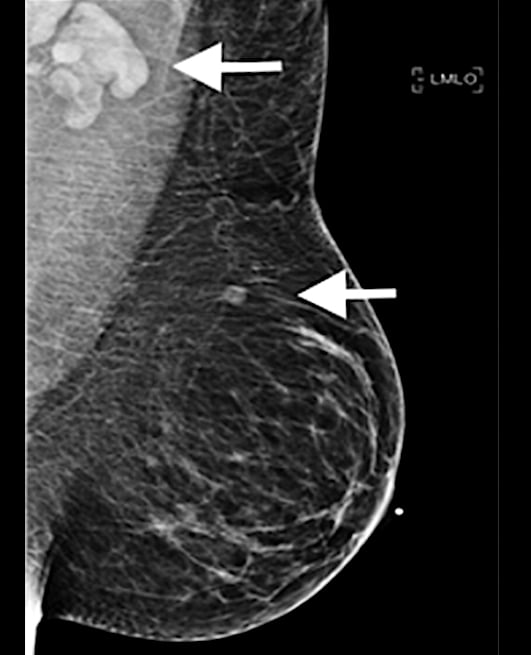
A 37-year-old woman developed a new, palpable left supraclavicular lymphadenopathy lump five days after her first dose of the Moderna COVID-19 vaccine in the left arm. On the day of vaccination, the patient was asymptomatic. This is an example of how the vaccine can mimic cancer and swollen lymph nodes. Read more about this case study. Image used with permission of RSNA.
While the mass COVID-19 vaccination effort over the past four months is bringing closer the light at the end of the pandemic tunnel, as with all things in medicine, it is not without a cost. But the cost here will be in terms of added patient anxiety and financial due to potential of additional, needed tests. There have been several alarms in peer-review literature and radiology societies the past couple months that the COVID-19 vaccines cause temporary inflammation and swelling of lymph nodes in some patients. While the experts say this is normal, it can be a major cause for concern if physicians, radiologists and patients are not aware of this vaccine presentation and assume it is a sign of infection or cancer, leading to additional diagnostic testing or followup exams.
Lymphadenopathy, also called adenopathy, is when lymph nodes are abnormal in size or consistency. The most common inflammatory type is lymphadenitis, producing swollen or enlarged lymph nodes. However, it is causing alarm on mammograms of recently vaccinated women and can lead to additional tests and imaging if found in patients who undergo CT scans for any reason.
The development of lymphadenopathy after being vaccinated for COVID-19 is a sign of the body's immune system gearing up in response to the vaccine and will go away, the experts say.[1,2] It has been seen with other vaccines for the influenza and human papillomavirus, but the two-dose Pfizer-BioNTech and Moderna COVID vaccines appear to affect a much larger number of people. In the clinical trials for the the Moderna vaccine, axillary swelling or tenderness was reported in 11.6% of patients (5% with placebo) after dose 1, and 16% (4.3% with placebo) after Dose 2.[3] This greatly concerns radiologist who look at mammograms and review exams looking for cancer, or monitoring cancer treatment in patients.
This concern prompted an editorial in the Radiological Society of North America (RSNA) journal Radiology: Imaging Cancer published April 9 that addresses the concerns and the diagnostic dilemma for patients, as lymphadenopathy can mimic cancer on imaging exams.[4] The authors of “COVID-19 Vaccination-Related Lymphadenopathy: What To Be Aware Of” point out that widespread patient education regarding vaccine-induced lymphadenopathy is needed. When vaccines are administered, side effects such as axillary swelling should be highlighted and normalized as an immune response initiated by the vaccine, they explain. The article also touches on the best times for patients to schedule imaging exams and offers follow-up recommendations.
“We write this editorial as a public service message at a time where other countries are starting mass vaccination programs with the goal of preventing unnecessary nodal biopsies and alleviating patient concern,” the authors wrote. “Imaging societies, clinicians and news media outlets should spread awareness to educate the public regarding this side effect to minimize patient anxiety.”
With mass vaccination rollout, the authors of the article said lymphadenopathy ipsilateral to the injected deltoid muscle has become an important manifestation of an immune response to be aware of as it may present as a diagnostic dilemma on cancer imaging studies. They said awareness will help the goal of preventing unnecessary nodal biopsies and alleviating patient concerns.
The article details the issue, what can be done and recaps the peer-review literature to date and guidelines issues since the start of vaccinations last December.
COVID Vaccine Can Lead to False Positive Mammograms
Breast imaging exams, screening mammography, breast ultrasound and breast MRI, can all show the presence of swollen lymph nodes. This has raised concerns and led to recommendations that women should be asked it they have received a COVID-19 vaccine prior to imaging exams. This prompted the Society of Breast Imaging (SBI) to quickly issue recommendations for when to image women who receive the COVID vaccine.[5] Recommendations were also published by women's health imaging specialists at Harvard Medical School and the Massachusetts General Hospital Department of Radiology.[6]
In a recent American Journal of Roentgenology (AJR) article, Shabnam Mortazavi, M.D., of the University of California at Los Angeles reviewed electronic medical records to identify women with post-COVID-19 vaccination adenopathy found in breast imaging between December 2020 to February 2021.[7] Twenty-three women exhibited axillary adenopathy ipsilateral to the vaccinated arm on screening or diagnostic breast imaging, and according to Mortazavi, “13% were symptomatic (axillary lump with possible tenderness).” Meanwhile, the adenopathy was detected incidentally on screening breast imaging in 43% (mammography, 5; ultrasound, 2; both mammography and ultrasound, 1; high-risk screening MRI, 2) and on diagnostic imaging for other reasons in 43% (BI-RADS 3 follow-up for breast finding, 3; screening callback for other reason, 2; non-axillary breast pain or lump, 5). Noting that the median interval between the first vaccine dose and imaging showing the abnormal node was 9.5 days, Mortazavi’s results counted a total of 57% of women with one abnormal node. BI-RADS 2 was assigned in one woman, BI-RADS 3 in 21 (ultrasound in 4–24 weeks), and BI-RADS 4 in one.
"There was a lot of confusion on the best way to manage these types of patients," said Constance "Connie" Lehman, M.D., Ph.D., lead author of the Mass General recommendations and chief of breast imaging, co-director of the Avon Comprehensive Breast Evaluation Center at the Massachusetts General Hospital, and professor of radiology at Harvard Medical School. "The first we tackled was a women who came in for a mammogram who had a recent COVID vaccination and had enlarged lymph nodes in the axilla on the same side she received the vaccine. So some were saying gosh, when we see this normally and we do not have a good explanation for this we are going to do a biopsy.Others said that is a bit to aggressive, lets bring them in for an ultrasound and we can do a short interval follow up until we show this is resolved and proving this is in fact not cancer, but just a temporary reaction to the vaccine. But, with the difficulties in getting patients to come in during COVID, we decided biopsies and short interval follow ups were not in our patients' best interest."
Instead, she said they based their recommendations for patients on the American College of Radiology (ACR) breast imaging and reporting recommendations, which state if you have a known inflammatory or infectious cause for unilateral axillary adenopathy, it is a benign BI-RADS 2 assessment.
"This has been reported over the decades with other vaccines, but it does seem the types of vaccines we are using for the COVID-19 virus prompts an even stronger response, which is why we are studying this so carefully," Lehman said. "We are carefully tracking this because we want to know how long this might persist on the mammograms or other imaging. We think 6 weeks is a reasonable estimate of how long we would expect to see enlarged lymph nodes on the mammogram, but we really don't know. We are in new territory now, so we are going to be carefully following patients. It could be up to two m months or more."
Should Imaging of Recently Vaccinated Patients be Delayed and for How Long?
Lehman said the Mass General guidelines and recently published paper included input from several imaging specialists in the department outside of breast imaging, because the concern of swollen lymph nodes run across all the subspecialties and may be found in other types of imaging. The rule of thumb they came up with for patients with enlarged lymph nodes who recently received the vaccine is to wait six weeks before following up with imaging exams if the swelling persists.
"For any imaging that is already scheduled, should they postpone or delay it, we break that down in the paper based on the reason for the exam," Lehman explained. There may be some screen tests that can be scheduled around the vaccine, or six weeks after that second dose. But in a lot of centers that would really put a lot of stress on an already extremely strained system, so we think people should interpret our guidelines locally and fit with their local resources."
Read More: https://www.ajronline.org/doi/abs/10.2214/AJR.21.25688
The SBI recommendations say that patients should schedule screening mammograms exams prior to the first dose of a COVID-19 vaccination, or 4-6 weeks following the second dose of a COVID-19 vaccination.
What Are the Side Effects of COVID-19 vaccination?
The Radiology: Imaging Cancer article authors said the most common COVID-19 vaccine side effects include local injection site pain, fever, chills, myalgias, headache and fatigue, with resolution usually in a few days.
There are three COVID-19 vaccines have been authorized by the U.S. Food and Drug Administration (FDA) for emergency use, the two-dose Pfizer and Moderna mRNA vaccines and the most recently authorized single dose Johnson and Johnson/Janssen adenovirus vector vaccine. Several other vaccines are in development or being distributed in other countries including those developed by Oxford-AstraZeneca (ChAdOx1 nCoV-19 or AZD1222), Gamaleya Research Institute of Epidemiology and Microbiology (Sputnik V), and the CanSinoBIO-Beijing Institute of Biotechnology (Convidicea or Ad5-nCoV). Through March 26, 2021, 526 million doses of vaccines have been distributed across the world.
Related COVID-19 Vaccine Radiology Content:
VIDEO: COVID Vaccine May Cause Enlarged Lymph Nodes on Mammograms — Interview with Constance "Connie" Lehman, M.D.
COVID-19 Vaccination Axillary Adenopathy Detected During Breast Imaging
PHOTO GALLERY: How COVID-19 Appears on Medical Imaging
CDC and FDA Call for Pause on Janssen COVID-19 Vaccine Due to Rare Blood Clots
Find more radiology related COVID content
References:


 April 23, 2024
April 23, 2024