Huge portions of the globally produced radiotracers find their origin within geographically centralized, commercial radiopharmaceutical manufacturing facilities or chemical laboratories. These facilities are limited in number and require expert supervision. There also is a considerable time gap between the preparation of these compounds and their transport and delivery to its end users at distant hospitals. The time between manufacture and delivery of radiotracers is an issue, because most tracers have a short half-life and their in-vivo performance degradation is accelerated with the passage of time. Half-lives of many agents are measured in hours, and this often limits imaging to morning hours after the delivery of the tracers.
The formulation of radiotracers can be both time- and task-intensive process. However the use of on-site, automated radiosynthesis systems may ease logistical concerns and facilitate the customized production of radiotracers. Within a decentralized setting for production and parameter control, multiple nuclear interface modules are engaged for the radiosynthesis process. This allows for the property adjustment of the compound to suit the specific application needed. The operation of these automated systems does not require expert technicians and can be used with only basic staff training. For this reason, it is more convenient for hospitals to use when they require high performance tracer compounds with specific in-vivo characteristics.
In recent years, there has been a significant increase in demand for radioactive tracer compounds in proportion to the progressive dominance of radiotherapy, PET and SPECT as effective diagnostic imaging techniques. This has spurred the demand for automated radiosynthesis modules.
The global automated radiosynthesis module market was valued at $20 million in 2015, and is expected to reach $32 million by 2022, supported by a compounded annual growth rate (CAGR) of 6.6 percent. The rise in incidences of life-threatening diseases among the global population has been the primary growth driver over the past decade.
The revised current good manufacturing practice (CGMP) regulations enforced by the U.S. Food and Drug Administration (FDA) is expected to channelize proper distribution of the products across the healthcare industry. The high cost of ownership of these systems is the major barrier to adoption. However, prices are expected to decline as competition among the manufacturers intensifies.
New Cardiac Perfusion PET Tracers
There have been concentrated research efforts over the past two decades to develop high-performance PET tracers. A recent study was conducted for the radiosynthesis and evaluation of a novel benzothiadiazole dioxide-based norepinephrine transporter PET-ligand [11C]Me@HAPTHI. Presynaptic norepinephrine transporter (NET) altercations are common to various neurological, neuropsychiatric, cardiovascular disorders. This calls for effective NET radioligands with excellent affinity and selectivity for PET imaging.
The new benzothiadiazole scaffold-based NET PET tracer exhibits both properties. However, it ranks lower on a scale of flexibility when compared to the previous versions of benzoimidazolones. The primary reason for the evaluation was to structure and optimize a small-scale radiosynthetic procedure for the production of [11C]Me@HAPTHI. The conditional parameters for optimized reaction were fed for automated radiosynthesis to GE’s Tracerlab FxC Pro synthesizer. Over the course of the study, the researchers worked on automating the procedure and increasing the scale of preparation. They also concluded that this discovery could possibly be improvised to formulate enhanced PET tracers.
The Basics of Cardiac Nuclear Imaging
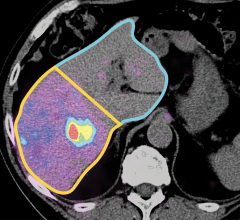
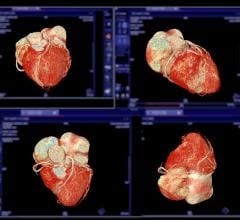
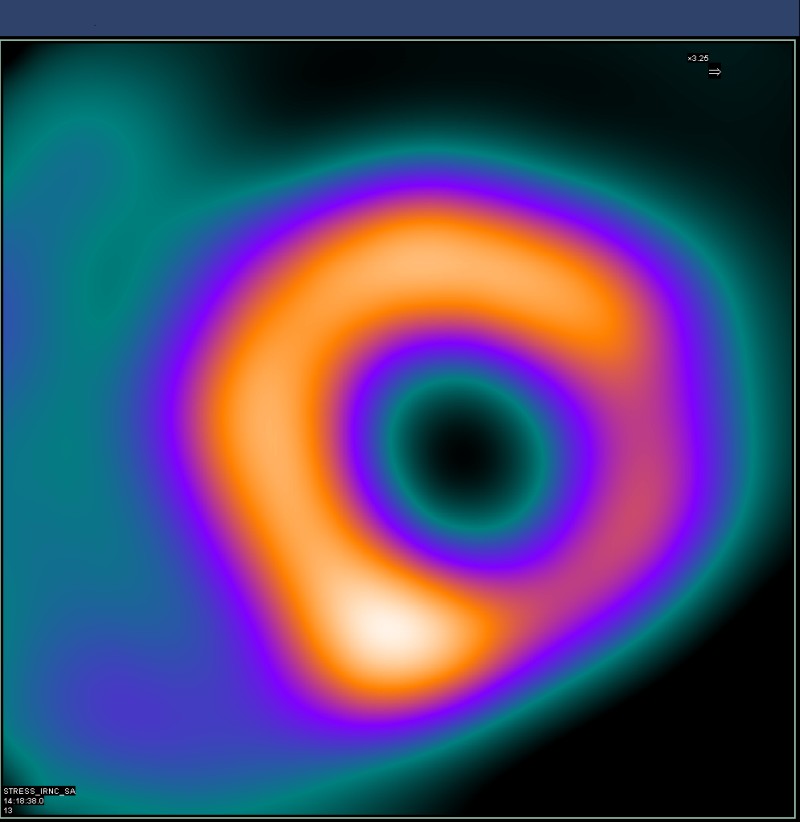
Noninvasive cardiac nuclear imaging shows the cellular metabolism of heart muscle cells during stress and exercise. This offers a good picture of the patient’s myocardial perfusion and is used as a gold standard in assessing cardiac ischemia.
The nuclear heart scan process involves intravenous injection of radioactive tracers, also called a radionuclide, which circulates in the blood and through the heart. A highly sensitive gamma camera is employed to capture still images and motion videos of the heart when it is under active stress or at rest. The procedure involves tomographic techniques, such as single photon emission computed tomography (SPECT) or positron emission tomography (PET), to detect the localized energy from radionuclides to generate pictures.
The tomographic scan is a useful reference for risk assessment in patients with signs of certain types of cardiovascular dysfunction. An electrocardiogram (ECG) is recorded for duration of the exam. The imaging can help identify abnormalities in coronary blood flow that cause myocardial infarction or ischemia.
Read the article “SNMMI Develops USP Recommendations for Compounded Sterile Radiopharmaceuticals.”
Read the article “Societies Call for Focus on Radiation Dose Optimization in Nuclear Cardiology.”
Editor’s note: Author Anamika Kumari is a content writer level II at Allied Analytics LLP. She has a bachelor’s degree in electrical engineering and is certified in industrial automation. Allied Analytics offers healthcare technology market research and business intelligence solutions.

 April 10, 2024
April 10, 2024