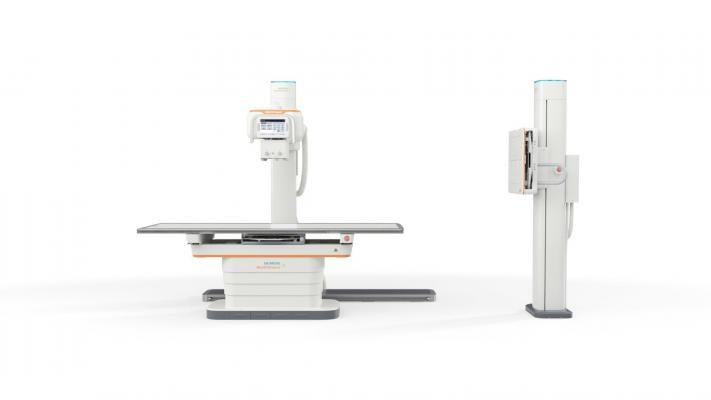
January 21, 2019 — The U.S. Food and Drug Administration (FDA) has cleared the Multix Impact, an affordably priced, floor-mounted digital radiography (DR) system from Siemens Healthineers that expands access to high-quality imaging and enhances the patient experience.
In addition to an intuitive operating system and versatile wireless detectors, the Multix Impact has a free-floating, flat tabletop for easy patient access. The touch user interface on the X-ray tube allows the technologist to remain at the patient’s side for longer periods of time, and when in the control room, the technologist can use the patient positioning camera to continuously monitor the patient, potentially reducing repeat imaging and avoiding excessive patient dose. The system also has an intuitive user interface with graphical program selection, as well as a patient positioning guide on the in-room touchscreen and control room workstation. Motorization and tracking features help to reduce the physical exertion of staff members.
For more information: www.usa.healthcare.siemens.com


 April 23, 2024
April 23, 2024