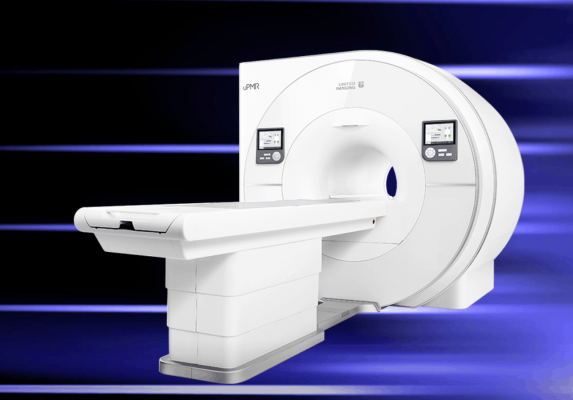
May 8, 2019 – United Imaging Healthcare (UIH), an international leader in advanced medical imaging and radiotherapy equipment, announced its U.S. Food and Drug Administration (FDA) clearance of the uPMR 790 HD TOF PET/MR. uPMR 790 redefines clinical routine imaging for PET/MR with the capability to scan a whole body within 20 minutes, balancing patient comfort with high-quality imaging. With the uPMR 790, United Imaging Healthcare demonstrates its commitment to advance precision medicine in the fields of neurology, oncology, and cardiology.
The next generation platform of the uPMR 790 delivers state-of-the-art PET and MR performance that rises above the current technology standards. The HD TOF PET platform is based on digital silicon photomultipliers (SiPMs) and lutetium-yttrium oxyorthosilicate (LYSO) crystals, offering a 2.8 mm NEMA PET spatial resolution with time-of-flight (TOF) technology and a large 32cm axial field-of-view. When this technology is combined with compressed sensing for whole body isotropic 3D MR imaging, it results in fast simultaneous whole-body PET and MR scans. This technology redefines PET/MR imaging by offering clinicians high isotropic spatial resolution to visualize small lesions while accelerating acquisition times to maximize patient comfort.
uPMR 790 also offers cutting-edge imaging for research, including theranostics and neuroscience which benefit from an increase in sensitivity, resolution, and coverage. United Imaging Healthcare’s uSync Research platform enables additional opportunities for research in areas such as simultaneous tracking of PET and MRI tracers, real-time cardiac PET/MR, functional neurological PET/MR, and multi-parametric radiometrics.
“The uPMR 790 sets a new standard for simultaneous PET and MR performance by not only enhancing the precision and resolution of scans, but by also dramatically changing the patient experience,” said Jeffrey M. Bundy, Ph.D., Chief Executive Officer of UIH Solutions. “Today’s announcement again delivers on our mission to embed breakthrough innovation across our portfolio. The uPMR is yet another proof point of our commitment to provide more patients in the United States with access to advanced technology and higher standards of care.”
For more information: www. usa.united-imaging.com
Related content:
FDA Clears United Imaging Healthcare uExplorer Total-Body Scanner

 April 17, 2024
April 17, 2024