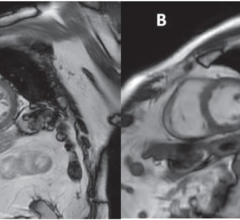
November 27, 2017 — At the 103rd Scientific Assembly and Annual Meeting of the Radiological Society of North America (RSNA), Siemens Healthineers debuted GOKnee3D, a magnetic resonance imaging (MRI) application that drastically shortens the time required to perform comprehensive diagnostic exams of the knee. Currently, a typical knee examination can be roughly 20 minutes. GOKnee3D enables a push-button, high-resolution diagnostic 3-D knee exam in just 10 minutes. The acquisition of high-resolution isotropic 3-D images subsequently allows flexible evaluation of the images in all possible planes, including double oblique and curved planar. Increasing MRI efficiency in this manner is especially important because knee examinations are the third most common type of MRI examination, accounting for 11 percent of all scans.
The volume acquisition of GOKnee3D is based on a CAIPIRINHA SPACE protocol, which enables higher scan speeds and optimal image reconstruction with better signal quality than in previous technologies. Supported by dedicated, high-channel knee coils as well as automated field-of-view adaptation based on machine learning and artificial intelligence, the MR scanner acquires the volume data of the knee joint at the touch of a button.
To develop and clinically validate the technique, Siemens Healthineers collaborated with Johns Hopkins University in Baltimore. “GOKnee3D enables comprehensive evaluation of internal derangement to the knee,” said Jan Fritz, M.D., assistant professor of radiology and radiological sciences at the Johns Hopkins University School of Medicine. “The fully automated CAIPIRINHA SPACE protocol provides high-quality MR imaging in 10 minutes and ensures consistency of image quality and operational efficiency. The high spatial resolution isotropic data sets help to visualize abnormalities with high accuracy, enable reformations of virtually any imaging plane, and create high-quality 3-D-rendered MR images.”
GOKnee3D is U.S. Food and Drug Administration (FDA) 510(k)-pending. It is available as an upgrade for the Magnetom Skyra 3T and Magnetom Aera 1.5T MR scanners, with eventual rollout planned for additional MR scanners.
For more information: www.usa.healthcare.siemens.com


 April 17, 2024
April 17, 2024