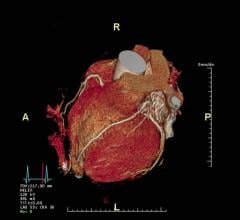
Siemens’ 510(k) pending GOBrain MRI application enables five-minute, clinically validated brain examinations with multiple orientations and all relevant contrasts. Image/courtesy Massachusetts General Hospital and Athinoula A. Martinos Center for Biomedical Imaging
November 29, 2015 — At the 101st Scientific Assembly and Annual Meeting of the Radiological Society of North America (RSNA), Siemens Healthcare introduced new applications designed to drastically reduce the time needed for magnetic resonance imaging (MRI) examinations of the brain. These new applications help increase patient throughput and potentially reduce costs per scan.
Brain scans account for roughly 20 to 25 percent of all MRI examinations, and fast examinations are essential for maintaining efficient workflow. The number of brain MRI examination is expected to grow in 2016, with an expected 45 million brain exams worldwide.
Siemens’ new Simultaneous Multi-Slice (SMS) application employs an innovative technique to acquire imaging slices simultaneously rather than sequentially – reducing 2-D acquisition times by up to a factor of 8. The length of advanced brain examinations can vary considerably, and now MR brain scans can be reduced to times compatible with the clinical routine (e.g., up to 68 percent for diffusion tensor imaging, or DTI). These advanced techniques will be applicable to patients with limited tolerance for longer scan times, such as geriatric or pediatric patients. SMS may be particularly beneficial in brain surgery cases through surgical mapping, potentially helping to reduce post-surgical deficits, and ultimately leading to improved efficiency in the utilization of OR resources.
Another new application, GOBrain, enables clinically validated brain examinations in just five minutes. Facilitated in part by Siemens’ high-channel density coils and the company’s DotGO MRI scanning software, the clinically essential image orientations and contrasts are acquired at the push of a button. Patient throughput is improved, and costs per scan can potentially be reduced. Shorter scan times are better tolerated by patients and can help reduce potentially costly and time-consuming rescans as well as potentially reduce sedation.
In addition to speed and quality, standardization across systems is also important for hospitals in meeting healthcare efficiency demands. With its syngo MR E11 software platform, Siemens introduces a uniform application platform for its Magnetom family of MR systems. The first systems to feature syngo MR E11 will be the Magnetom Aera 1.5T and Magnetom Skyra 3T systems. The focus, in addition to expanding the application offering, is achieving consistency across the entire fleet of scanners and managing them effectively. A single consistent user interface, as well as intuitive protocol optimization enabled by the DotGO scanning software, further aids users in providing standardization and reproducibility.
The syngo MR E11 software platform and applications are also designed for Siemens’ Biograph mMR positron emission tomography (PET)/MR scanner. New BodyCompass technology is designed to enable motion-free PET images with MR-based motion compensation beyond gating, which could be particularly beneficial in delineating abdominal and lung lesions, which are prone to motion.
The SMS and GOBrain applications are currently pending U.S. Food and Drug Administration (FDA) 510(k) approval. syngo MR E11 for Biograph mMR is currently under development.
For more information: www.siemens.com



 April 24, 2024
April 24, 2024