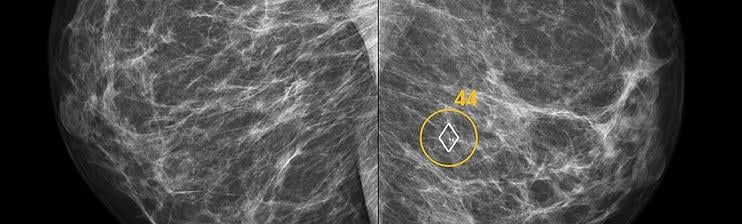
November 28, 2018 — ScreenPoint Medical announced it has received 510(k) clearance from the U.S. Food & Drug Administration (FDA) for Transpara detection and decision support software, designed to assist radiologists with the reading of screening mammograms.
Transpara is the first FDA 510(k)-cleared artificial intelligence (AI) application for detecting breast cancer in screening mammograms, based on its functionality that interactively provides support for detection and diagnosis. The clearance was supported by the results of a multi-reader, multi-case reader study, which demonstrated that radiologists significantly improved detection accuracy when using Transpara for decision support without increasing reading times. In the study, the stand-alone sensitivity and specificity of Transpara was nearly at the same level as that of radiologists. The full results of the study, “Detection of breast cancer using mammography: Impact of an Artificial Intelligence support system,” were recently published in Radiology.1
In the editorial, “Will AI Succeed Where Traditional CAD Failed?”, published as a companion to the reader study, Manisha Bahl, M.D., wrote: “The results of the study by Rodríguez-Ruiz and colleagues suggest that integration of AI systems into routine clinical practice could help radiologists with varying levels of training and experience achieve performance benchmarks and therefore improve the quality of mammography across the country.”
Transpara already has European regulatory approval (CE Mark) for use with mammography and digital breast tomosynthesis (DBT) images from multiple vendors and is installed at leading breast imaging centers in Europe. Transpara DBT is still investigational in the U.S.
ScreenPoint will showcase Transpara and its integration with multiple vendor workstations at the 104th Annual Radiological Society of North America (RSNA) meeting, Nov. 25-30, 2018 in Chicago. Two studies demonstrating that radiologists significantly improved cancer detection in mammography when using Transpara while maintaining workflow will also be presented at RSNA:
- “Detecting Breast Cancer in Mammography: A Deep Learning-Based Computer System versus 101 Radiologists”; and
- “Improving Radiologists' Breast Cancer Detection with Mammography Using a Deep Learning-Based Computer System for Decision Support.”
Utilizing state-of-the-art image analysis and deep learning technology, Transpara automatically identifies soft-tissue and calcification lesions and combines the findings of all available views into a single cancer suspiciousness score. Breast imaging professionals can use this Transpara Score to automatically identify exams that are highly likely to be normal and the exams with features indicating highly increased risk of cancer. Interactive decision support is provided to improve assessment of lesions. Information is provided concurrent during reading and only when needed. As a result, it does not slow down reading.
For more information: www.screenpoint-medical.com
Reference
1. Rodriguez-Ruiz A., Krupinski E., Mordang J.J., et al. Detection of Breast Cancer with Mammography: Effect of an Artificial Intelligence Support System. Radiology, Nov. 20, 2018. https://doi.org/10.1148/radiol.2018181371


 April 18, 2024
April 18, 2024 








