January 20, 2022 — Leading health tech firm Qure.ai has gained 510(k) clearance from the Food and Drug Administration (FDA) for an artificial intelligence (AI) algorithm called qXR-BT, that will help doctors in assessing Breathing Tube (BT) placements. Through chest X-rays, the algorithm assists clinicians for intubated patients in locating the BT placement and automating measurements. This is the first solution of its kind to automate the manual measurement process for both endotracheal and tracheostomy tubes.
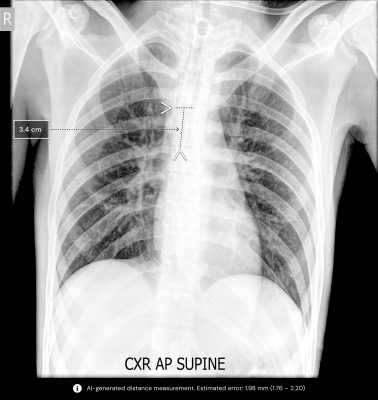
Verification of endotracheal tube (ETT) placement is imperative for the oxygenation, ventilation, and airway protection of your patient. While a common procedure done in hospitals daily, Rates for incorrect ET tube placement have been noted to be up to 25%1. According to the Society of Airway Management, mistakes during the intubation process threaten the lives of 121,000 hospital intensive care unit patients annually in the United States2. Even if you have an expert clinical team inserting and securing the tube after the initial placement, ETT migration is an inevitable consequence of coughing, suctioning, transport, and patient movement.
Qure's qXT-BT algorithm analyzes the tube position, automates measurement, and gives the physician a report on the tube’s positional accuracy in less than a minute. This enables clinicians to rapidly identify if the tube is properly positioned or whether extra attention is required. The algorithm is vendor-agnostic and is designed to work on both portable and stationary X-ray machines.
Mannudeep Kalra, M.D., attending thoracic radiologist, Massachusetts General Hospital and Professor of Radiology, Harvard Medical School, who was involved in a research collaboration evaluating the technology said, “Daily monitoring of Tubes is critical for all intubated inpatients, and sometimes an arduous task on the portable exam with either the carina obscured or the tip not visible. An accurate AI solution could be a valuable aid for reporting on these chest X-rays- especially with the measurement.”
“We are pleased to have received FDA clearance for qXR-BT. In the last two years, we have seen the need to decrease processing times and solve workflow delays,” said Prashant Warier, CEO and Founder, Qure.ai. “Especially in the wake of the COVID-19 pandemic and the need for mechanical ventilation in affected patients, the need for prompt assistance to an overburdened healthcare workforce is paramount,” he added.
qXR-BT is expected to become a standard feature of any critical care framework, giving residents and junior clinicians more confidence in reliably measuring breathing tube placement in intubated patients.
For more information: www.qure.ai


 April 17, 2024
April 17, 2024 








