August 22, 2018 — Philips recently announced the introduction of the Epiq CVx cardiovascular ultrasound system. Built on the Epiq ultrasound platform, Epiq CVx is specifically designed to increase diagnostic confidence and simplify workflow for clinicians, giving them more time to interact with their patients and reducing the need for repeat scans. According to Philips, 95 percent of a group of clinicians who were shown the new system believed it offered improved image quality: sharper and clearer images [1]. Philips is also introducing the Epiq CVxi, specifically tailored for use in the interventional lab. Both systems are CE marked and have received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
“The EPIQ CVx brings together advanced image quality, quantification and intelligence specifically for the cardiologist,” said Roberto Lang, M.D., professor of medicine, director, Noninvasive Cardiac Imaging Laboratory at the University of Chicago Medicine. “I was impressed with the TrueVue feature, which elevates 3-D ultrasound imaging to a totally new level and could impact diagnostic ability of echocardiography in different clinical scenarios, like better understanding of the anatomy of mitral valves.”
As pressures on healthcare systems around the world continue to increase, cardiologists have more patients to examine in less time, according to Philips. By using advanced 3-D organ modeling, image slicing and proven quantification, the company said its anatomical intelligence is helping make ultrasound exams easier to perform and more reproducible.
Philips described the Epiq CVxi as a third-generation integrated ultrasound-angiography cath lab solution for real-time, workflow-optimized image guidance and advanced quantification for structural heart procedures.
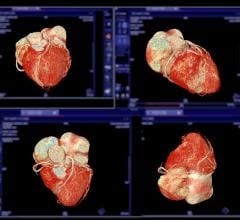
Debuting at the European Society of Cardiology (ESC) 2018 annual meeting, Aug. 25-29 in Munich, Germany, the Epiq CVx includes higher processing power, exceptional image clarity and sharpness, improved exam efficiencies, and more robust and reproducible quantification, enabled by anatomical intelligence. The system includes TrueVue, giving clinicians the ability to see photorealistic renderings of the heart, which improves cardiac anatomy analysis by offering detailed tissue and depth perception imaging through a new virtual light source. It provides cardiologists with high image quality through the latest generation OLED monitor, offering a more dynamic, wider viewing angle for side-by-side image comparison.
The system offers a variety of new features including Dynamic Heart Model. Building on Philips HeartModelA.I., it uses anatomical intelligence to automatically quantify left ventricle function to produce a multi-beat analysis for adult patients. Dynamic Heart Model has been shown to reduce the amount of time to generate a 3-D ejection fraction, an important measurement in determining how well the heart is pumping out blood, by 83 percent [2]. It also delivers a high level of robustness and reproducibility, even for patients with an arrhythmia. The systems also includes the new S9-2 PureWave Transducer, which simplifies pediatric cardiac imaging exams by displaying high levels of detail and contrast resolution through the single-crystal technology. It also provides tissue information at greater depths and enhances pediatric capability for coronary artery visualization.
The Epiq CVx includes a cardiology-specific user interface that simplifies the exam experience through a user-configurable touch-screen interface, allowing clinicians to personalize their controls and improve workflow for their cardiology exams. The system features strong security capabilities and protocols.
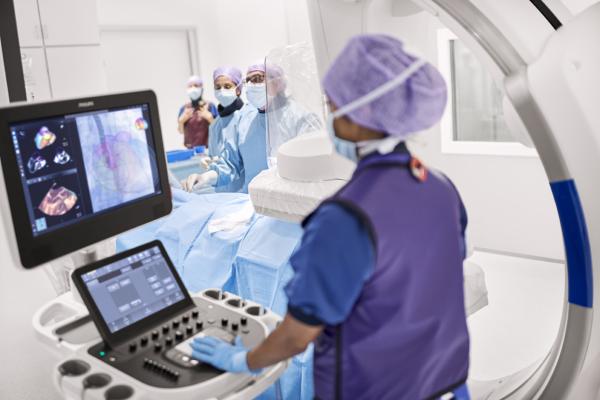
The Epiq CVxi with EchoNavigator, designed specifically for use in the cath lab, is Philips’ third-generation interventional solution to streamline communication between the interventional cardiologist and the echocardiographer during complex interventional exams. Combining live ultrasound and X-ray information into one intuitive view, EchoNavigator helps interventional cardiologists oversee procedures along with the location of key anatomical structures. In addition, MultiVue provides more flexibility when using 3-D during diagnostic or interventional procedures as the clinician can see multiple and flexible views at once.
For more information: www.usa.philips.com/healthcare
References
[1] Results obtained during user demonstrations performed in December 2017 with the Epiq CVx and the iE33 systems. The research was designed and supervised by Use-Lab GmbH, an independent and objective engineering consultancy and user interface design company. The tests involved 42 clinicians from 17 countries. The various types of cardiac customer segments represented were adult diagnostics and interventional, adult diagnostics, and pediatric diagnostics and interventional.
[2] Prado A, Narang A, Volpato V, Kumari N, Prater D, Addetia K, Patel AR, Mor-Avi V, Lang RM: Automated dynamic measurement of left heart chamber volumes for quantification of ejection and filling parameters. JASE 31(6):B111; 2018


 April 08, 2024
April 08, 2024