May 25, 2017 — NucleusHealth has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Nucleus.io technology.
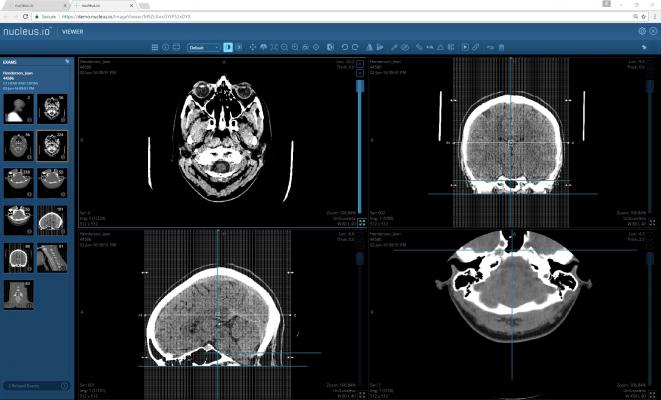
Nucleus.io is a web-based, scalable and secure cloud platform for medical image management. At the core is patented streaming technology that provides diagnostic workstation performance in a web browser. Nucleus.io can improve collaboration and patient care via fast access to images, reduce information technology (IT) overhead, is optimized for machine learning, and provides flexibility and customization through numerous RESTful APIs.
NucleusHealth plans to commercialize the new technology in software-as-a-service (SaaS) modules and a platform-as-a-service (PaaS) to allow the healthcare industry to take full advantage of cloud and streaming technologies without having to simultaneously decommission their current enterprise image management solutions. Initial modules include:
- Cost-effective cloud storage of images with fast web retrieval of the data;
- Seamless exchange of imaging studies between health systems, caregivers and patients; and
- A fully diagnostic, browser-based workstation for integration with electronic medical record (EMR) systems.
In addition to the preliminary SaaS modules, if health systems or healthcare companies have clinical applications requiring the integration of medical images, they can benefit from deploying their applications on the Nucleus.io PaaS. NucleusHealth also plans to launch a fully cloud-based image management SaaS solution for health systems seeking to replace their aging picture archiving and communication system (PACS).
For more information: www.nucleushealth.io


 April 22, 2024
April 22, 2024