June 1, 2016 — Alzeca Biosciences announced successful preliminary results for ADx, its novel, proprietary diagnostic imaging technology. The technology is designed to enable diagnosis of Alzheimer’s Disease for the first time on conventional magnetic resonance imaging (MRI), providing results a decade or more before the onset of symptoms of cognitive decline.
Successful development of ADx would make early and reliable Alzheimer’s testing available to millions, using scanning technology that is widely available, and at far lower cost and without the radiation of current positron emission tomography (PET) scans. Earlier detection of Alzheimer’s could revolutionize the treatment of the disease and vastly improve the efficiency of clinical trials for new therapies by identifying appropriate candidates for participation.
Alzeca also is exploring the significant additional potential of the underlying platform of ADx, which can be modified to bind to different targets in the brain. With these modifications, the platform could be used to image and diagnose other serious neurodegenerative diseases, and ultimately be used as a drug delivery system for treating those conditions.
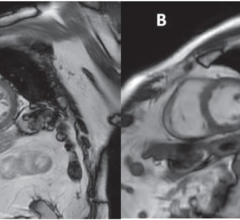
In peer-reviewed studies, the ADx nanoparticle demonstrated the ability to carry an encapsulated agent across the blood-brain barrier to bind precisely to amyloid plaques in the brains of test mice. Researchers were then able to obtain precise, high-resolution images of those plaques using ordinary MR equipment. These results, along with successful preliminary toxicity studies, allow Alzeca to accelerate the development and testing of ADx, with the goal of beginning human clinical trials in 2018. In May, the company was awarded a $322,000 grant from the Alzheimer’s Drug Discovery Foundation (ADDF) to fund the next phase of testing of ADx in canine subjects.
More than 27 million people suffer from Alzheimer’s disease today, and it is the sixth leading cause of death in the United States. By 2050, the disease could result in a $1.1 trillion annual cost to the U.S. healthcare system. Yet there is no cost-effective, readily available, reliable way to diagnose the disease before the onset of cognitive decline – which is when current Alzheimer’s therapies have traditionally been most effective.
ADx has the potential to confirm an Alzheimer's diagnosis, as well as to rule out such a diagnosis. Misdiagnosed dementia patients not only receive the wrong therapies, but the cost of treating a misdiagnosed patient adds $14,000 to the annual cost of treatment.
Alzeca’s biodiagnostic platform is a nanoparticle that can carry an imaging marker or other “payload” and pass through the normally impenetrable blood-brain barrier. The outside of each nanoparticle is designed to bind precisely to specific target substances in the brain, delivering the payload to that target.
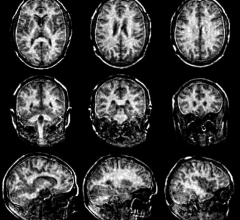
ADx is the first product based on the platform. ADx carries an MRI agent as its payload and through a ligand targets the amyloid plaques that are the hallmark of the onset of Alzheimer’s disease, developing ten or more years before cognitive impairment. Once ADx is bound to the plaques, beta-amyloid, the hallmark of the disease in its earliest stages, can be imaged in high resolution using a standard MRI scan.
MR scans are radiation-free, far less costly and more readily available throughout the world compared with the only current alternative, a PET scan. PET scans cost two to five times MRI scans and require radioactive imaging agents that preclude routine use, while ADx would permit physicians to track the patient’s condition over time and even screen for the disease.
For more information: www.alzeca.com


 April 18, 2024
April 18, 2024