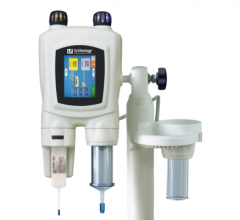
July 24, 2008 – Covidien recently launched the Optistar Elite contrast delivery system, designed to inject contrast media-related drugs into a patient’s vascular system to aid in obtaining diagnostic images when used with magnetic resonance (MR) imaging equipment.
The product recently received FDA approval and already carries a CE Mark.
The Optistar Elite aims to provide improved performance and ease of use, compared with the previous Optistar LE model.
Among the features on the Optistar Elite injector is patency check, which aids in preventing extravasation, assists in reducing the potential of certain medical errors and helps enhance clinical and work flow efficiency. Aimed at improving patient comfort, care and safety, the patency
check feature enables healthcare providers to confirm an unobstructed, or patent, fluid pathway to the patient prior to injection of contrast media-related drugs. A visual alert on the injector powerhead and on the control console also warns clinicians when the programmed pressure limit engages due to a blockage during the patency check.
The Optistar Elite, combined with the use of Covidien’s Ultraject prefilled syringes for contrast media-related drugs, can help decrease the risk of certain medication errors that may be caused by manually filling syringes. Designing the Optistar Elite to be compatible with the Ultraject prefilled syringes also eliminates syringe preparation and labeling steps, which helps meet certain regulatory requirements of the Joint Commission.
In addition, the powerhead buttons on the Optistar Elite injector are color-coordinated with the Ultraject prefilled syringes to help avoid medication errors resulting from the use of the wrong syringes and to help ensure proper procedural set up, while a status line banner guides technologists through each step of the set up process.
For more information: www.covidien.com


 April 12, 2024
April 12, 2024