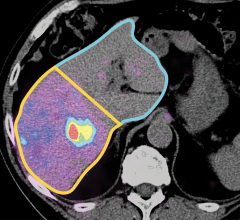
May 28, 2019 — A new clinical study shows that Natera's Signatera test identified colorectal cancer recurrence up to 16.5 months earlier than radiologic imaging by detecting traces of tumor DNA in the blood after surgery. The test also identified patients most likely to relapse, both before and after chemotherapy.1 Results were published in the May issue of JAMA Oncology.1
The prospective, multicenter study enrolled 130 patients with stage I–III colorectal cancer from Aarhus University, Randers and Herning hospitals in Denmark. The study used Natera's Signatera research-use-only test to evaluate molecular residual disease (MRD) in 829 blood samples collected serially throughout the patient monitoring period.
Results demonstrated the Signatera test detected molecular recurrence up to 16.5 months earlier than standard-of-care radiologic imaging (average 8.7 months). Serial testing picked up 14 out of 16 relapses (patient-level sensitivity 88 percent), and among patients who did not relapse, 455 out of 456 post-surgical blood samples correctly tested negative (test-level specificity 99.8 percent).
The study also found that MRD status was the most significant predictor of relapse after adjusting for all other known risk factors, including disease stage and lymph node status. Signatera MRD-positive patients who did not receive treatment relapsed in 93 percent of cases. Among patients who remained MRD-negative, the relapse rate was 3 percent. These results underscore the potential of MRD status to risk stratify patients more accurately after surgery to determine which patients need additional therapeutic interventions and which could be safely monitored.
"Our study showed unequivocally that Natera's personalized multiplex PCR-based next-generation sequencing is a highly sensitive approach for detecting molecular residual disease in the blood," said Claus Lindbjerg Andersen, M.Sc., Ph.D., study lead investigator, Aarhus University. "The results show the potential of blood-based MRD detection to drive a paradigm-shift in how patients are managed during the course of their disease."
The study also reported the first published demonstration of Natera's plasma-based whole exome sequencing capability, in which there was strong concordance between whole exome results from the plasma and tumor biopsy at time of metastasis.
For more information: www.natera.com
Reference
1. Reinert T., Henriksen T.V., Christensen E., et al. Analysis of Plasma Cell-Free DNA by Ultradeep Sequencing in Patients With Stages I to III Colorectal Cancer. JAMA Oncology, published online May 9, 2019. doi:10.1001/jamaoncol.2019.0528


 April 17, 2024
April 17, 2024