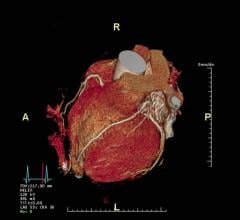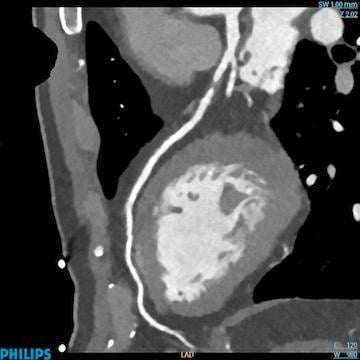
June 17, 2016 — Three medical professional societies this week jointly released a new reporting system to standardize reporting of patients undergoing coronary computed tomography angiography (coronary CTA). Termed CAD-RADS (Coronary Artery Disease Reporting and Data System), this system will bring consistency to reporting of coronary CTA diagnostic information for millions of patients worldwide.
Unlike many other major disease areas, until now no standardized system has existed to classify and report patient data for CT scans of coronary artery disease. CAD-RADS fulfills that long-sought goal of the radiology, cardiology and industry communities.
The suggested CAD-RADS classification is applied on a per-patient basis and represents the highest-grade coronary artery lesion documented by coronary CTA. It ranges from CAD-RADS 0 (zero) for the complete absence of stenosis and plaque to CAD-RADS 5 for the presence of at least one totally occluded coronary artery, and should always be interpreted in conjunction with the impression found in the report. Specific recommendations are provided for further management of patients with stable or acute chest pain based on the CAD-RADS classification.
Ricardo C. Cury, M.D., Miami Cardiac and Vascular Institute, Radiology Associates of South Florida and SCCT past president, led a 17-member multi-disciplinary Expert Consensus Group representing four professional societies: The Society of Cardiovascular Computed Tomography (SCCT, the lead society for CAD-RADS), The American College of Radiology (ACR, co-author), the North American Society for Cardiovascular Imaging (NASCI, co-author) and the American College of Cardiology (ACC, which endorsed this publication).
Watch a video interview with Cury on what CAD-RADS means for cardiology and radiology.
Cury noted that "our societies developed CAD-RADS to improve communication of coronary CTA results to referring physicians in a consistent fashion, including considerations for patient management. Standardized reporting will benefit education, research, peer review and quality assurance and improved quality of care. The teamwork amongst our multi-society, multi-disciplinary writing group was exemplary."
Cury urges the practice community and industry to become familiar with the CAD-RADS classification and modifiers system. "Today's article is the starting point for a very important process. Next, to promote incorporation of CAD-RADS into daily practice, SCCT will partner with other societies and industry to develop tools for every coronary CTA facility considering adoption of CAD-RADS."
SCCT President-Elect Leslee J. Shaw, Ph.D. added that the next step will be "development of highly sophisticated decision support tools and registries. CAD-RADS is an important part of our strategic plan to ensure that all patients undergoing CCTA have the highest quality of cardiovascular care."
ACR Chief Executive Officer William T. Thorwarth Jr., M.D., FACR, commented that "The ACR, through our representatives on this important project, was pleased to be a part of this critical effort to enable the standardized reporting and subsequent ongoing evaluation and optimal performance of this valuable examination."
The document was published online in the Journal of Cardiovascular Computed Tomography, the Journal of the American College of Radiology (JACR) and JACC Cardiovascular Imaging.
For more information: www.scct.org


 April 23, 2024
April 23, 2024