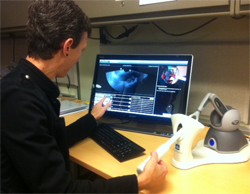
November 11, 2011 – MedaPhor, the United Kingdom-based ultrasound simulation company, announced that the University of Washington Medical Center in Seattle is the first United States hospital to install its revolutionary ScanTrainer ultrasound simulation system.
ScanTrainer is a virtual “real feel” ultrasound training simulator that provides students with a 24/7 educationally driven training program that replicates the real scan experience. Trainees can literally “feel” what they see on the computer screen, since they hold a Sensable Phantom haptic device instead of a mouse as they progress through the training. ScanTrainer allows users to develop the complex mix of cognitive skills and eye-hand movement coordination, but without the need for an ultrasound machine, a patient and considerably reduced direct supervision by an expert.
The University of Washington Medical Center is one of North America’s leading hospitals and the system has been installed in their Institute for Simulation and Interprofessional Studies (ISIS). ISIS is a leading center in the use of simulation technologies to improve the quality of health care education and improve patient safety and outcomes.
Thomas J. Benedetti, M.D., M.H.A., professor of obstetrics and gynecology and director of education in obstetrics and gynecology said, “We saw the Scan Trainer as breakthrough technology. It will allow us to come closer to our goal of having trainees acquire technical skills in a realistic simulated environment before performing them on patients. To date, the acceptance and enthusiasm of the trainees and faculty has been very satisfying.”
For more information: www.medaphor.com


 April 08, 2024
April 08, 2024 








