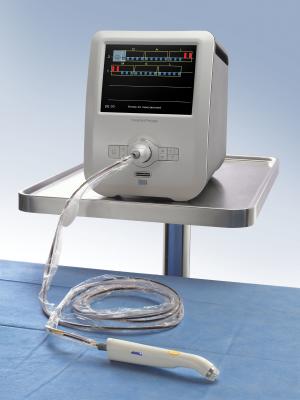
April 7, 2016 — Dune Medical Devices has received pre-market approval (PMA) from the U.S. Food and Drug Administration (FDA) to make modifications to MarginProbe, an FDA-approved device for real-time detection of cancer in breast conserving surgery. The modifications will incorporate new features that will enable the device to be compliant with the recent changes in Restriction of Hazardous Substances (RoHs) requirements necessary to sell into the European Union (EU) market.
The new upgrade includes manufacturing MarginProbe 1.2 to meet changes in the RoHs requirements, which are expected to be adopted by the United States in the near future. In addition, Dune Medical Device equipped the instrument with a new, updated screen for displaying the results and an upgraded operating system.
Margin Probe 1.2R also contains a much stronger and more modern central processing unit (CPU), allowing the medical device to support anticipated evolutions in both technology and operating systems.
Traditionally, one in four women who undergo a lumpectomy procedure require a re-excision to remove cancer missed during the first procedure. That number is reduced by 51 percent when MarginProbe is used during the initial procedure.
For more information: www.dunemedical.com


 April 18, 2024
April 18, 2024 








