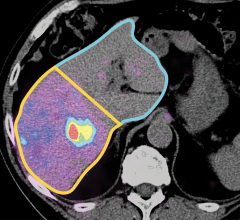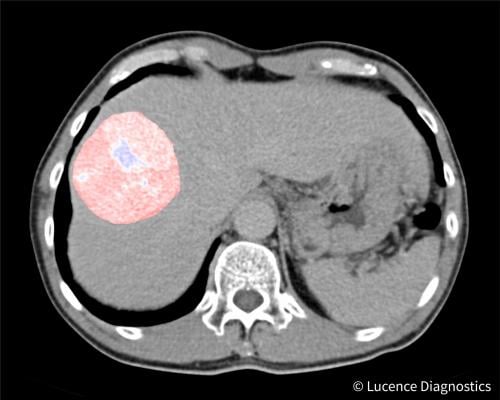
Pseudocolor accentuated CT scan image of a liver tumor. Image courtesy of Lucence Diagnostics.
March 12, 2019 — Genomic medicine company Lucence Diagnostics announced a new project to develop artificial intelligence (AI) algorithms for improving diagnosis and treatment of liver cancer. The goal is to combine the imaging and molecular data from liver cancer patients into smarter software tools that help physicians make better treatment decisions.
Lucence will be working with Olivier Gevaert, Ph.D., assistant professor of medicine (biomedical informatics) and of biomedical data science at the Stanford University School of Medicine. Having developed LiquidHallmark, which it calls the world's first liquid biopsy next-generation sequencing test that analyzes the DNA of cancer-causing mutations and viruses, Lucence will contribute its genomics expertise and proprietary sequencing technology to this project.
Liver cancer is the second leading cause of avoidable cancer deaths globally1, and hepatitis viruses contribute to the bulk of this disease. The incidence rate of liver cancer is also rising faster than any other cancer in both men and women in the United States2. The best chance of cure is surgery, and good characterization of the extent and type of the disease is critical for surgical planning. Imaging tests such as ultrasound, computed tomography (CT) and magnetic resonance imaging (MRI) play a crucial role in the visualization of liver tumors. Fusing imaging data with sequencing data that includes both cancer mutations and viral DNA will create a unique opportunity for AI-based approaches to advance liver cancer care.
This project will evaluate a dataset of more than 5,000 patients to identify image changes and patterns that are linked to diagnostic and treatment outcomes in liver cancer.
For more information: www.lucencedx.com
References
1. Knaul F.M., Arreola-Ornelas H., Rodriguez N.M., et al. Avoidable Mortality: The Core of the Global Cancer Divide. Journal of Global Oncology, Aug. 10, 2018. DOI: 10.1200/JGO.17.00190.
2. Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA: A Cancer Journal for Clinicians, Jan. 8, 2019. DOI: 10.3322/caac.21551.


 April 17, 2024
April 17, 2024