May 27, 2015 — Ipsen announced the signature of an agreement to acquire OctreoPharm Sciences, a private German life sciences company developing innovative radioactive-labeled compounds for molecular imaging diagnostics and therapeutic applications. Ipsen plans to maintain the company location and staff to ensure successful transition of know-how and expertise. The company expects to complete its acquisition once closing conditions have been cleared.
Under the terms of the agreement, which is subject to closing conditions, OctreoPharm’s shareholders are eligible to receive up to a total of approximately €50 million for the purchase of 100 percent of the company’s shares in the form of an upfront payment and downstream payments contingent upon clinical and regulatory milestones.
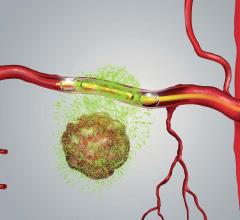
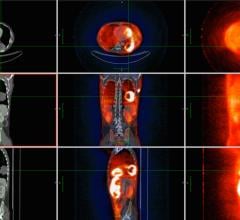
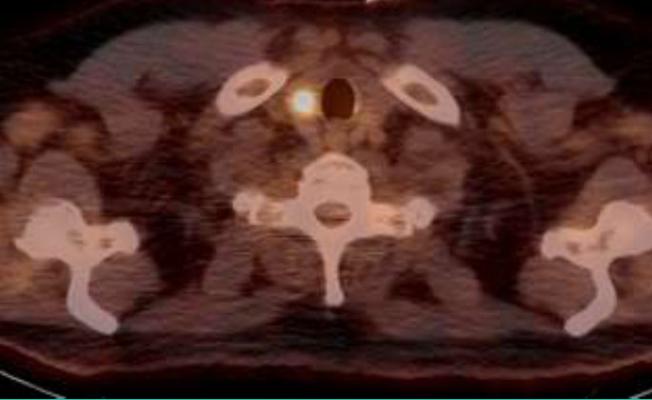
The transaction fits into Ipsen’s strategy to extend the scope of its portfolio in neuroendocrine tumors (NET). OctreoPharm is developing an innovative theranostic approach for the management of NET based on a somatostatin receptor antagonist peptide. The therapeutic compound is a tumor cell-selective somatostatin antagonist peptide labeled with Lutetium-177 (Lu-177) for use as ‘peptide receptor radionuclide therapy’ (PRRT) for the treatment of neuroendocrine tumors, and is currently in preclinical development. The diagnostic compound is an NET imaging tool utilizing positron emission tomography/computed tomography (PET, PET/CT), and is currently in clinical development.
The acquisition includes an agreement with Eckert and Ziegler, one of OctreoPharm’s shareholders, to provide contract manufacturing services for the radio-labeling of the therapeutic compound.
For more information: www.ipsen.com, www.octreopharm.com


 April 05, 2024
April 05, 2024