June 13, 2019 — Ikonopedia will showcase its suite of structured breast reporting and Mammography Quality Standards Act (MQSA) management tools designed to improve reporting efficiency, and optimize facility operations at two upcoming radiology meetings. The company will be exhibiting at the Society for Imaging Informatics in Medicine (SIIM) annual meeting, June 26-28 in Aurora, Colo., and the Association for Medical Imaging Management (AHRA) annual meeting, July 21-25 in Aurora.
Ikonopedia recently introduced an automated, closed loop resolution manager with integrated pathology reporting capabilities to make sure that patient findings do not get lost in the shuffle of a busy clinic.
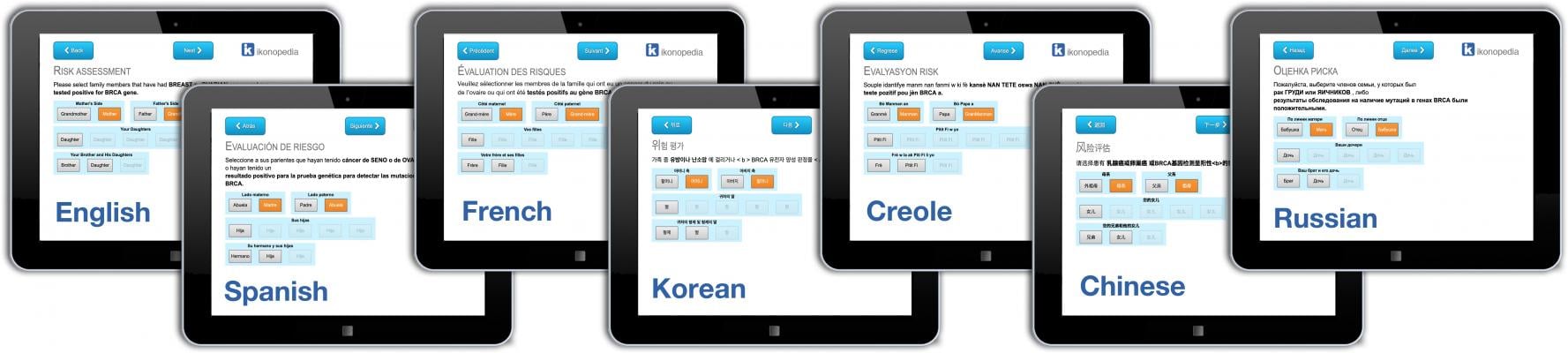
To expand access to quality breast healthcare, Ikonopedia also recently introduced an integrated risk assessment tool, now available in any language. The company also provides users with the first-ever web-based version of the Tyrer-Cuzick Breast Cancer Risk Assessment Tool. Risk data is used to create alerts for the radiologist, populate the clinical section of the report and automatically update the patient letter. A high-risk patient alert identifies patients with a 20 percent or greater lifetime risk and information about the score is instantly viewable.
For more information: www.ikonopedia.com


 April 18, 2024
April 18, 2024 








