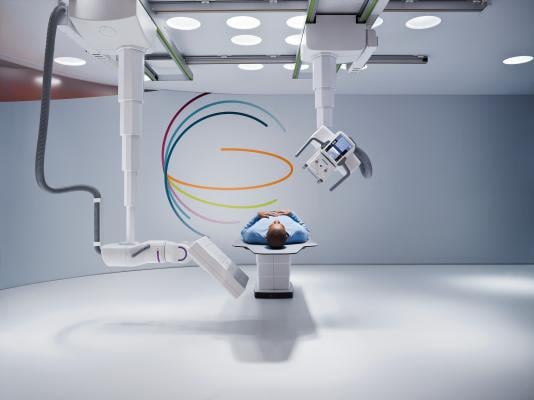
February 11, 2016 — The new Multitom Rax robotic X-ray device could revolutionize the digital radiography space and allow X-ray devices to compete with more advanced imaging modalities, said an analyst with research and consulting firm GlobalData.
Developed by Siemens Healthcare, the Multitom Rax was cleared by the U.S. Food and Drug Administration (FDA) in November 2015 and is expected to launch in the United States in April 2016.
According to Sarah Janer, GlobalData’s analyst covering medical devices, the Multitom Rax can perform a wide range of diagnostic examinations including fluoroscopy, radiography, angiography and orthopedic scans, giving it an edge over the current generation of devices.
Janer explained: “As digital radiography has traditionally been limited by 2-D imaging and a lack of flexibility, the Multitom Rax is designed to overcome those barriers and offers the distinctive precision of medical robotics.
“The device offers a high level of precision and flexibility, and could be used as a partial substitute for CT [computed tomography] scanners. Furthermore, it has a long lifespan, and Siemens has engineered the device to be adaptable to future technological trends.”
GlobalData forecasts that the U.S. market for digital radiography X-ray devices will expand from $644 million in 2015 to reach $942 million by 2020, expanding at a Compound Annual Growth Rate (CAGR) of 7.9 percent. However, the introduction of robotic advanced X-ray technology could significantly boost future growth.
The analyst also noted that while other digital radiography models, such as GE Healthcare’s Discovery XR656 Plus and Toshiba’s RADREX-i, offer a great amount of flexibility and function, there is currently no competition in the robotic advanced X-ray field.
Janer concluded: “Once Siemens receives clearance for the Rax’s 3-D applications, GlobalData expects that robotic advanced X-ray instruments will become a hospital standard, and competition in the field will increase as hospitals look to replace their outdated digital radiography models with superior, more universal devices.”
For more information: www.usa.healthcare.siemens.com


 April 09, 2024
April 09, 2024