March 9, 2017 — Piramal Imaging SA and Isologic Innovative Radiopharmaceuticals recently announced that Health Canada has issued a Notice of Compliance (NOC) to the latter for NeuraCeq (florbetaben F-18 injection). Isologic has received marketing authorization from Health Canada for the commercial production and market supply of NeuraCeq in Canada.
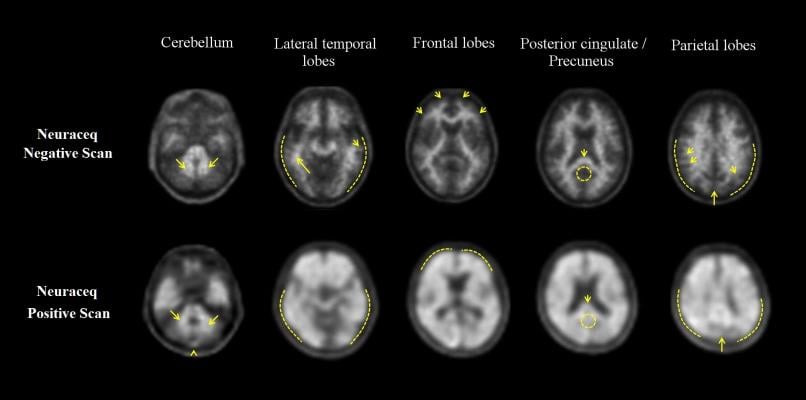
NeuraCeq is the first diagnostic radiotracer to support the early diagnosis of Alzheimer's disease (AD) that is commercially available in the country. NeuraCeq availability in Canada was previously limited to investigational medicine purposes.
"The commercial availability of NeuraCeq will provide physicians throughout Canada a non-invasive method to more confidently and accurately assess complex and atypical cases of cognitively impaired patients for early diagnosis of Alzheimer's disease," said Jean-Paul Soucy, M.D., medical director of PET (positron emission tomography) imaging at the Montreal Neurological Institute. "In the absence of an approved disease modifying treatment, advancing our ability to make an early and accurate AD diagnosis is critically important to providing optimal symptomatic treatment and non-pharmacological measures to manage disease progression and quality of life in these patients."
NeuraCeq has previously received approval from the U.S. Food and Drug Administration (FDA) and several countries in the European Union (EU) and Asia. It is a diagnostic radiotracer that when used in combination with PET imaging, can identify beta-amyloid plaques in the human brain, which are known as an important biomarker for Alzheimer's disease.
For more information: www.piramal.com/imaging

 April 10, 2024
April 10, 2024 




![(A) PET images of [68Ga]Ga-DOTA-ZCAM241 uptake at baseline and 3, 7, and 12 days after injection as inflammatory arthritis developed in single representative individual mouse. Images are normalized to SUV of 0.5 for direct comparison between time points. (B) CD69 immunofluorescence Sytox (Thermo Fisher Scientific) staining of joints of representative animals during matching time points.](/sites/default/files/styles/feed_medium/public/PET%20Tracers.jpeg?itok=P5Di6MIe)