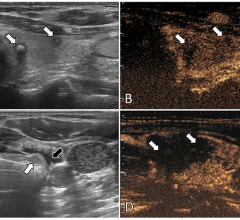
June 26, 2019 — The U.S. Food and Drug Administration (FDA) issued the final guidance: Marketing Clearance of Diagnostic Ultrasound Systems and Transducers. This final guidance provides detailed recommendations for manufacturers seeking marketing clearance of diagnostic ultrasound systems and transducers.
This final guidance:
-
Provides recommendations on information that should be included in a premarket notification (510(k) submission) for diagnostic ultrasound systems and transducers;
-
Describes the types of modifications to a diagnostic ultrasound device for which the FDA does not intend to enforce the requirement to submit a new premarket notification (510(k) submission); and
-
Introduces a new transducer element integrity check which applies to all the ultrasound devices covered in the guidance.
On Aug. 22, 2019, the FDA will host a webinar for device manufacturers and others interested in learning more about this final guidance.
Read the full guidance document here.
For more information: www.fda.gov


 April 08, 2024
April 08, 2024