February 18, 2019 — Mirada Medical Ltd announced U.S. Food and Drug Administration (FDA) 510(k) clearance for Simplicit90Y. Designed in collaboration with BTG to facilitate personalized treatment for patients with liver cancer, Simplicit90Y is an easy-to-use dosimetry software developed for accelerating dosimetry planning and improving Y90 transarterial radioembolization (TARE) workflow.
Watch the VIDEO: Y90 Embolization of Liver Cancer at Henry Ford Hospital
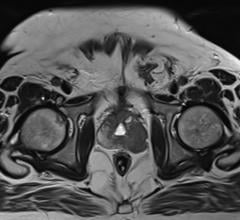
Simplicit90Y provides digital processing, review and reporting of medical images, including planar scans (static, whole body) and tomographic scans acquired by gamma cameras or positron emission tomography (PET) scanners. The software is intended for use by interventional radiologists or nuclear medicine physicians, with unique options for data display, quality control, image manipulation and quantification analysis. Together, these features allow for more standardized and reproducible dosimetry planning and post-treatment validation, and the ability to personalize the treatment of each patient.
BTG will be at The Society of Interventional Radiology (SIR) 2019 congress, March 23-28 in Austin, Texas.
The FDA 510(k) clearance follows Simplicit90Y’s CE Mark certification and approval for use in Canada.
For more information: www.mirada-medical.com

 April 16, 2024
April 16, 2024