December 7, 2018 — iCAD Inc. announced clearance by the U.S. Food and Drug Administration (FDA) for their latest, deep-learning, cancer detection software solution for digital breast tomosynthesis (DBT), ProFound AI. The solution built on artificial intelligence (AI) is now available to healthcare facilities in the U.S.
The FDA clearance is based on positive clinical results from a large reader study completed earlier this year and presented at the 2018 Radiological Society of North America (RSNA) annual meeting, Nov. 25-30 in Chicago. The research was performed with 24 radiologists who read 260 tomosynthesis cases both with and without iCAD’s ProFound AI solution. The findings show increased cancer detection rates, reduced false positive rates and patient recalls, and a significant decrease in interpretation times.
“This technology shows tremendous promise in assisting radiologists in detecting cancers, reducing recalls and increasing efficiency when reading tomosynthesis studies,” said Emily Conant, M.D., professor and chief, Division of Breast Imaging, vice chair of faculty development, Department of Radiology at the Hospital of the University of Pennsylvania. “Clinical data shows that when tomosynthesis readers use the ProFound AI algorithm, case-level sensitivity is improved by 8 percent on average and reading times are significantly decreased. Radiologists with various levels of expertise may benefit from this AI-driven technology when reading large tomosynthesis data sets.”
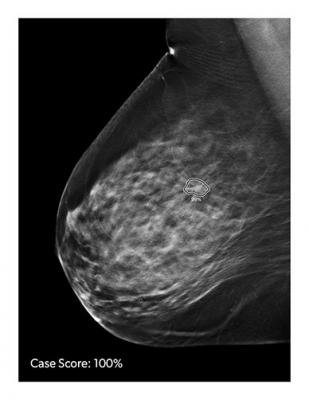
ProFound AI is a high-performance, deep-learning, cancer detection and workflow solution for DBT, delivering improvement of cancer detection rates by an average of 8 percent and decreasing unnecessary patient recall rates by an average of 7 percent. The new technology is trained to detect malignant soft-tissue densities and calcifications. It also provides radiologists with scoring information representing the likelihood that a detection or case is malignant based on the large dataset of clinical images used to train the algorithm.
In addition to improving clinical performance related to breast cancer detection and false positive rates, study results showed ProFound AI can reduce radiologists’ reading time by more than 50 percent on average. An increase in reading time has been a significant challenge for radiologists when moving from 2-D to 3-D mammography.
The solution is currently available for use with - DBT systems in the U.S., Canada and Europe.
For more information: www.icadmed.com


 April 18, 2024
April 18, 2024 








