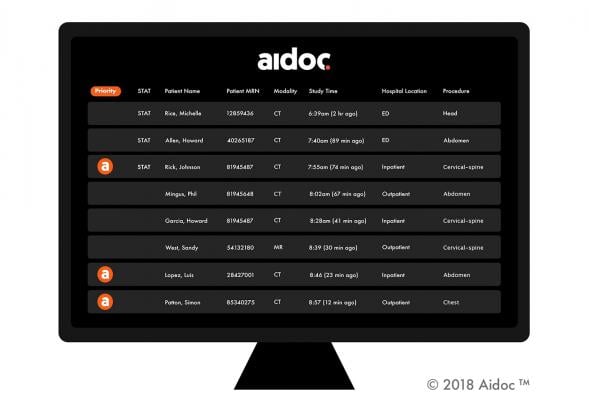
May 15, 2019 — Artificial intelligence (AI) solutions provider Aidoc has been granted U.S. Food and Drug Administration (FDA) clearance for an additional product in its expanding suite of AI-based workflow orchestration solutions. The clearance is for Aidoc's Pulmonary Embolism (PE) solution that works with radiologists to flag and triage PE cases in chest computed tomography (CT) scans. The approval comes just weeks after Aidoc closed a $27 million funding round, bringing its total funding to $40 million.
"In addition to the significant value provided to the department by Aidoc's ICH [Intracranial Hemorrhage] solution, we recently added the PE module to the workflow," said Barry Pressman, M.D., chair of imaging at Cedars-Sinai Medical Center. "I was impressed by the fact that the coverage continuously grows, allowing us to add this product in the workflow of more radiologists, becoming part of our daily work. I was also pleased by the ability of the software to prioritize PE studies accurately."
In the United States alone, up to 200,000 people a year die due to PE.1 Undetected or late-detected PE is one of the most common causes of preventable death in hospitalized patients. PE diagnosis can be highly challenging due to its variable and non-specific presentation, making AI-driven workflow triage especially beneficial. Recent research published at the 2019 European Congress of Radiology (ECR) in Vienna2 further shows the accuracy and value Aidoc's solution can provide, according to the company.
"It is clear that AI will play a tremendous role in the future of radiology," said Daniel J. Durand, M.D., chair of radiology at LifeBridge Health, Baltimore. "Considering the complexity of vascular diagnosis, we are eager to see how Aidoc's solutions can benefit our pulmonary embolism patients and bring tomorrow's technology to LifeBridge Health today."
Aidoc's solutions analyze medical images directly after the patient is scanned and notify the radiologists of cases with suspected findings, to assist with prioritization of time-sensitive and potentially life-threatening cases. Aidoc cuts the time from scan to diagnosis for some patients from hours to under five minutes, speeding up treatment and improving prognosis.
For more information: www.aidoc.com
References
1. Tarbox A.K., Swaroop M. Pulmonary embolism. International Journal of Critical Illness & Injury Science, published online March 22, 2013. DOI: 10.4103/2229-5151.109427


 April 17, 2024
April 17, 2024 








