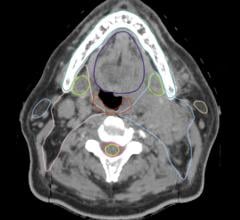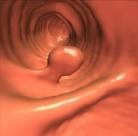
Computed tomography colonography, also known as virtual colonoscopy.
June 3, 2010 - Positron emission tomography (PET) combined with computed tomography colonography (CTC) may provide a suitable alternative for detecting polyps and cancer in the colon and does not require sedation or bowel preparation, according to a study published in the June issue of The Journal of Nuclear Medicine.
Authors of the study, “Nonlaxative PET/CT Colonography: Feasibility, Acceptability, and Pilot Performance in Patients at Higher Risk of Colonic Neoplasia,” researched the effectiveness of using a combination of CTC, an imaging technique that is less invasive alternative to the standard colonoscopy test, and PET scans without any bowel preparation to detect significant abnormalities in the colon. The current study is the largest to date investigating combined PET CTC in patients without any bowel preparation.
Fifty-six patients agreed to undergo a one-hour CTC and PET scan without the use of laxatives two weeks before their scheduled colonoscopy. Patients then completed a questionnaire to see how they tolerated the tests and which one they preferred.
The colonoscopy results were then compared with the CTC scan on its own and with the CTC and PET scans combined. The study found that the combined PET CTC scans detected all the important larger polyps found by the invasive colonoscopy technique. In addition, most patients found the combined scan technique more comfortable and preferred it to colonoscopy.
“The work has shown that combined PET CTC is technically feasible, well tolerated by patients and capable of achieving high diagnostic accuracy,” said Stuart A. Taylor, M.D., University College London, and lead researcher of the study. “This test would be mainly used in patients less able to tolerate invasive investigations or the preparation required if physicians want to exclude any major pathology in the colon and abdomen.”
Reference: Taylor, S., Manpanzure, L., et al. “Nonlaxative PET/CT Colonography: Feasibility, Acceptability, and Pilot Performance in Patients at Higher Risk of Colonic Neoplasia.” The Journal of Nuclear Medicine. June 2010.
For more information: www.snm.org


 April 16, 2024
April 16, 2024