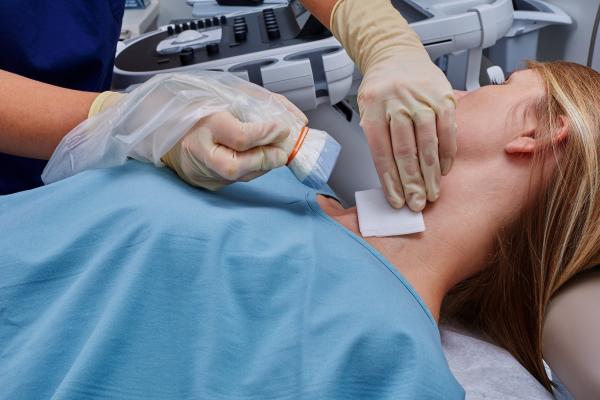
June 6, 2019 — Civco Medical Solutions announced a new solution that removes gel from ultrasound procedures. Envision viral barriers allow for 100 percent gel-free procedures.
Envision ultrasound probe covers and scanning pads are activated with a sterile liquid and require no gel. This reduces the risk of contamination, can improve the quality of fine needle aspiration (FNA) specimens and simplifies clinician workflow.
Ultrasound gel can pose a contamination risk in certain procedures, and multiple clinical studies have shown sterile ultrasound gel to be the cause of healthcare-associated infections. Studies report that gel can:
-
Contribute to increasing nosocomial infection, the spread of hospital acquired infections1,2,3,4
-
Cause bacteria to be introduced into the blood stream3,6
-
Make the disinfection of sterilization process of devices, including ultrasound probes, less effective5
Utilizing Envision during needle-guided interventions — including central venous catheter (CVC) and peripherally inserted central catheter (PICC) procedures, or while scanning non-intact skin — eliminates the need for gel and helps reduce the risk of contamination.
Gel is also commonly used during an ultrasound-guided fine needle aspiration (FNA) procedure. However, clinical studies have shown the adverse effects ultrasound gel can have on FNA specimens:
-
Impairs the visibility of cells and interferes with staining of cells7
-
Causes a significant increase in the number of slides with artifacts7
-
Causes widespread cell lysis, increasing the risk of misinterpretation and a false positive diagnosis8
-
Mimics colloid, creating difficulty in differentiation between artifact and colloid7,9
Envision covers can help improve the visibility of cells in FNAs by removing gel from these procedures.
Envision viral barriers are available in two configurations: an ultrasound probe cover and a scanning pad. Envision probe covers can be used during ultrasound-guided needle interventions, and the Envision pad adheres to the patient or the ultrasound probe to scan non-intact or sensitive areas, including on neonates or post-surgical wound sites.
The probe cover and scanning pad enable physicians and clinicians to simplify workflow throughout a procedure:
-
A quick-peel, silicone liner adheres safely to the probe face or to the patient's skin — no gel needed inside the cover or applied to the patient
-
Apply a sterile liquid, such as sterile saline, to the Envision cover or pad, or directly on the patient's skin, and begin scanning
-
Pre-cleaning of the ultrasound probe is simplified without gel, before sending to high-level disinfection
Envision is approved by the U.S. Food and Drug Administration (FDA) for use in the United States, and available for sale in numerous countries around the globe.
For more information: www.civco.com
References

 April 24, 2024
April 24, 2024