November 13, 2017 — Barco will host presentations at the 2017 Radiological Society of North America (RSNA) annual meeting, Nov. 26-Dec. 1 in Chicago, to address the pressing challenges of diagnostic accuracy and workflow efficiency in radiology. The company will demonstrate how its technologies help hospitals, radiologists and information technology (IT) managers improve performance and cost containment in this rapidly evolving era in healthcare.
Barco is hosting a series of booth talks designed to arm organizations with real-world solutions to their most pressing radiology IT challenges. Sessions include:
- Assuring compliance of the healthcare enterprise — With the proliferation of reading environments and changing regulatory requirements, it can be difficult to ensure compliance across all locations. Barco will demonstrate how to improve quality assurance/quality control via an automated, online management of all flat panel display assets.
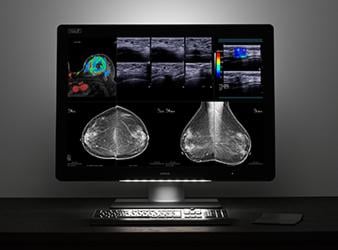
- Important considerations for multimodality breast imaging — Learn how to transform breast imaging workflow to improve efficiency and working comfort while reading the multitude of imaging modalities, from 2-D and 3-D mammography to magnetic resonance imaging (MRI) and ultrasound (US). Attendees will see how bringing all exams onto a single screen improves comparison in accordance with new ACR-AAPM-SIIM Practice Parameter Determinants of Image Quality in Digital Mammography.
- Optimizing lifecycle management of diagnostic displays — Barco will also help attendees maximize return on investment (ROI) and ensure perpetual compliance by understanding the critical lifecycle factors affecting display investment. See how an online quality assurance/quality control (QA/QC) service can automatically track and manage display compliance, conduct data analytics, support decisions and improve lifecycle planning of assets.
Barco is also providing displays for the Image Perception Lab, funded by the National Institutes of Health (NIH) National Cancer Institute, Brigham and Women’s, and Harvard Medical School. Barco will also conduct an experiment on “The effect of color rendering alternatives on detectability of clinically relevant features in PET-CT (positron emission tomography/computed tomography).”
Barco will present its portfolio of mammography and general radiology displays, designed to reveal the subtlest details and improve precise detection at the earliest stages of disease, according to the company.
The company’s optimized display systems enhance reading speed and accuracy, with many featuring intuitive workflow tools and, all supported by Barco’s automated QA and compliance solution, MediCal QAWeb.
The Coronis Uniti is the only multimodality breast imaging display that also allows viewing of picture archiving and communication system (PACS) images, according to Barco. Radiologists can view grayscale and precisely calibrated color, and clearly see subtle details to improve clinical decision-making. Complete studies can be conducted without multiple monitor setups, boosting workflow efficiency and reducing visual fatigue.
The Coronis Fusion 6MP is designed as a workhorse for multimodality imaging. The brightest 6MP available, it delivers superior image quality while increasing productivity, according to Barco.
The Nio Color 3MP brings hospital-quality imaging to radiologists’ home office desktops, helping clinicians discern even tiny details, thanks to high brightness (500 cd/m2) and contrast ratio.
For more information: www.barco.com


 April 22, 2024
April 22, 2024