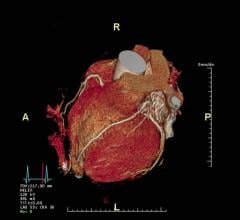
3D Systems’ D2P FDA-cleared software allows clinicians to 3-D-print diagnostic patient-specific anatomic models. Image courtesy of 3D Systems.
September 12, 2019 — 3D Systems has received additional U.S. Food and Drug Administration (FDA) 510(k) clearance for its D2P software allowing clinicians to 3-D print diagnostic patient-specific anatomic models.
D2P (DICOM-to-Print) relies on automatic segmentation tools driven by deep learning that allow medical practitioners to quickly create accurate, digital 3-D anatomic models from medical imaging data. With the additional FDA clearance, D2P addresses the growing demand by point-of-care (POC) institutions for in-house manufacturing using an accurate and reliable 3-D segmentation solution that can produce 3-D-printed models.
D2P now also includes the latest advancements in deep learning image processing technology and virtual reality (VR) visualization. This allows hospitals and device manufacturers to significantly reduce the time associated with the creation of 3-D models.
The software also includes a volumetric VR solution enabling instant views of patient scans in a 3-D environment — facilitating surgical planning and conversations between medical staff and their patients.
"We are used to going into surgery with uncertainties and an arsenal of contingency plans," said Solomon Dadia, M.D., deputy director of the orthopedic-oncology department and director of the 3D surgical center at Souraski Medical Center in Tel-Aviv. "With 3-D-printed models and enhanced 3-D visualization tools such as VR, we are able to gain a better understanding of the surgery and pathology we are going to treat. This allows us to come up with a more precise surgery plan designed to deliver a better surgical outcome."
In accordance with the FDA announcement on new guidelines for 3-D-printed, patient-specific anatomic models in 2017, diagnostic-quality models must be an output of a Class II regulated medical device software. According to the company, 3D Systems is the only company to offer both a software solution and compatible printers of its own that meet this regulatory requirement. Anatomic models can be produced using a variety of 3D Systems printing technologies — ColorJet Printing, MultiJet Printing, Stereolithography and Selective Laser Sintering — including materials that are capable of sterility and biocompatibility.
For more information: www.3dsystems.com


 April 23, 2024
April 23, 2024