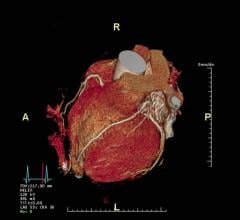
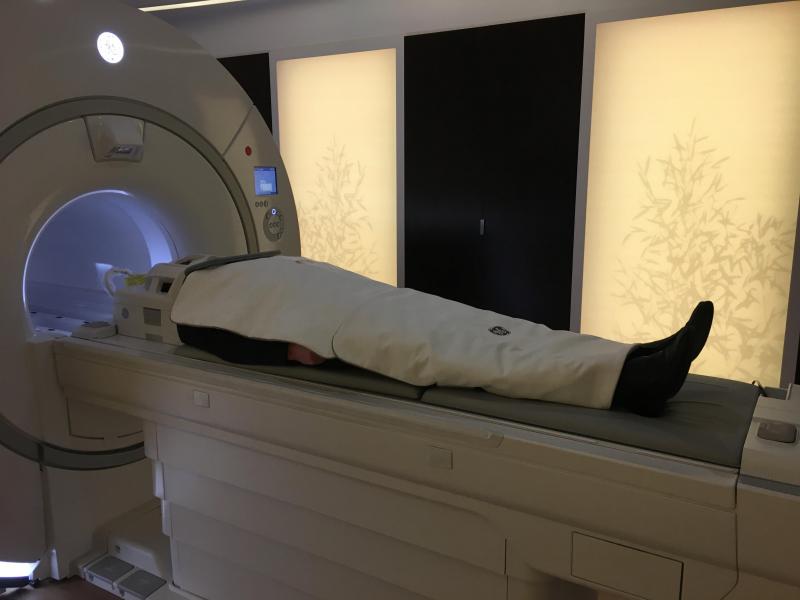
The Philips IQon Elite debuted at RSNA 2017 with features designed for emergency and trauma care, as well as oncology. Image courtesy of Philips Healthcare
The tenets of value-based medicine rippled across the exhibit floor like waves from a stone tossed in a pond. But it was the stone, metaphorically comprised of artificial intelligence, that got most of the attention. Its presence was everywhere at the 2017 Radiological Society of North America (RSNA) meeting — in demonstrations and on booth placards; on a banner above the sky bridge connecting the McCormick Place Towers; and in a “Machine Learning Showcase” on the exhibit floor.
The message of RSNA 2017, however, was not about artificial intelligence (AI) as much as it was efficiency, effectiveness, patient centricity and cost savings.
Merger Mania
Waves were made on the RSNA exhibit floor by more than just AI and value-based medicine. The Canon/Toshiba merger made a splash with the two, and Toshiba subsidiary Vital Images all appeared in large side-by-side booths. Registration issues forced the separate booths — Canon at one end, Toshiba in the middle and Vital Images at the other. But those issues will go away Jan. 4, 2018, after which, Toshiba equipment will be Canon branded (although the Vital Images brand will continue.)
There were signs of other mergers. The union of Lexmark’s Perceptive Business Unit with Hyland Software in 2017 consolidated Perceptive’s enterprise imaging products and Hyland’s OnBase content management software. Change Healthcare replaced longtime RSNA exhibitor McKesson, following the merger of Change and McKesson Technology Solutions.
And more mergers and acquisitions could be coming. Through mid-November, investors poured $122 million into AI startups, according to market research firm Signify Research. Already at RSNA 2017, some AI startups had entered formal partnerships with large vendors, for example, RadLogics with Agfa HealthCare and Zebra Medical with Change.
An initial public offering of Siemens’ healthcare unit, the Healthineers, could fuel acquisitions, as well. The IPO is expected to happen in 2018.
AI – The Enabler
Value-based medical technologies enabled by artificial intelligence stood out. Among them was a work-in-progress 3-D camera from Siemens that uses smart algorithms to ensure patients are positioned in the isocenter of the computed tomography (CT) scanner. The FAST (Fully Assisting Scanner Technologies) 3-D camera, which could begin shipping commercially by the end of 2Q 2018, may reduce the variability of results and, consequently, the need for rescans.
“This FAST 3D camera is really supporting precision medicine,” said André Hartung, head of Siemens CT. “For each individual patient, the exact right position is done automatically.”
GE demonstrated a smart algorithm that alerts medical staff to a potentially catastrophic abnormality. The work-in-progress, built into GE’s Optima AMX portable X-ray machine, is being groomed to scour postoperative radiographs for signs of a collapsed lung (pneumothorax). If one is found, a red indicator at the bottom of the onboard display lights up to alert the technologist.
“In addition to that, you can make sure these images are prioritized to the top of the queue for the radiologist to read,” said Michelle Edler, global president and CEO of GE Healthcare X-ray.
Units with the embedded algorithm could begin rolling off GE production lines in 4Q 2018.
For the second RSNA meeting in a row, Phillips showcased artificially intelligent tools for fetching and interpreting data. Smart algorithms built into Philips’ Illumeo product assess the context in which the radiologist is working. Illumeo can then call up tools, gather data and make relevant measurements that promise to help radiologists make diagnoses.
Watch the VIDEO "Deep Learning is Key Technology Trend at RSNA 2017" where Freiherr and ITN Editor Dave Fornell explain how AI is being incorporated into medical imaging.
Watch an interview with Adam Flanders, M.D., chair of the RSNA Radiology Informatics Committee, in the VIDEO "How Utilization of Artificial Intelligence Will Impact Radiology."
See several examples of how AI is being implemented in radiology in real products
IT Advances Healthcare
High-quality care requires fast action. One way to streamline the diagnostic process is to prioritize the interpretation of patient cases. Carestream Health’s Worklist Orchestrator does so by factoring in different criteria. These include the availability and expertise of the interpreter; required turnaround times; urgency of cases; and referring physicians’ preferences for specific interpreters. Criteria are weighted to reflect the wants and needs of the provider, according to Carestream’s worldwide product line manager, Tomer Zonens.
“We want our customers to speak to us about what is most important; about what they value,” Zonens said.
At RSNA 2017, Nuance framed its PowerShare network as a bridge for sharing images among radiologists. Initially created to transmit text created using the company’s dictation technology, the network is being leveraged by Nuance to transmit and receive images across the enterprise.
Konica Minolta expanded its Exa enterprise imaging solution with new components, including a performance dashboard. This board displays such metrics as daily exam volume, radiologist performance and ordering preferences of referring physicians. Exa can also assemble the data into customized reports.
Fujifilm Medical Systems USA unveiled the ability to do advanced visualization from within its Synapse 5 PACS. The capability, according to William Lacy, Fujifilm vice president of medical informatics, makes advanced visualization simpler and more efficient.
Need For Increased Speed in MRI
Magnetic resonance imaging (MRI) scans take too long. Siemens’ GOKnee3D, which is pending U.S. Food and Drug Administration (FDA) clearance, promises to cut the time for knee scans in half. Using the new Siemens technology, MR knee scans might be done in 10 minutes at 3T and 11 minutes at 1.5T.
GOKnee3D is not the first such Siemens accelerator. It was preceded at RSNA 2015 by GOBrain, which cut the time for brain MR to five minutes.
Siemens prioritized the development of these technologies partly on the basis of procedure volume. Knee exams comprise the third most common MR scan (after brain and spine), accounting for about 11 percent of the 6.73 million done on Siemens MR systems, according to a 2016 company survey. Siemens engineers did not develop an accelerator package for spine MR, which represents the second most common MR scan, because of technical challenges, according to Daniel Fischer, Siemens head of clinical marketing for MRI. But the company has not given up.
“We’re looking at just about any region that lends itself to a ‘GO’protocol,” Fischer said.
Following FDA clearance, GOKnee3D is expected to appear first as an upgrade for Siemens’ high-end Skyra 3T and Aera 1.5T scanners. Eventually it will be rolled out for other MR scanners in the company’s portfolio.
Streamlining MR scans has long been a priority among equipment vendors. It began in earnest in 2005 with Philips’ launch of parallel imaging. The Dutch company coined the term SENSE (short for sensitivity encoding). The technology has since evolved into Compressed SENSE, which Philips claims can reduce scan times up to 30 percent. The technology, which is pending FDA clearance, can be used “to do all types of scans and all anatomies,” said Martijn Hartjes, Philips’ head of MR product marketing.
Phonetically – and operationally – similar is Siemens’ Compressed Sensing. The company initially positioned Compressed Sensing as the means to eliminate patient breath holds during cardiac MR exams. At RSNA 2017, Siemens described the use of Compressed Sensing in abdominal MR to eliminate breath holds (and, consequently, multiple acquisitions.)
Also using a variation of the Philips moniker is GE’s HyperSense. This technology, which has been shipping since summer 2017, allows brain scans on the company’s 3T Premier, for example, in under five minutes, according to GE.
At RSNA 2017, GE showed its proprietary AIR (Adaptive Imaging Receive) technology as the foundation for lightweight RF coils. The AIR coils were configured into rectangular pads that looked like blankets. They – and others in development – are 60 percent lighter than conventional coils, according to the company.
Three coils built around AIR Technology have been designed for use on GE’s top-of-the-line Signa Premier 3T scanner: head coil, anterior array extending 65 cm (25.6 inches) and a posterior array extending 110 cm (43.3 inches). GE said the technology will begin shipping in 2018.
Thomas McGuinness, GE Healthcare vice president and chief strategy and commercial officer, said AIR coils exemplify the company’s focus on “innovations that not only improve the resolution of images but also the experience for everyone involved in the process.” Their light weight and flexibility make positioning and handling easy.
New MRI Scanners
Philips unveiled a 1.5T scanner called the Prodiva. Philips MR executive Hartjes said the system addresses the coming reality of value-based medicine, specifically the need to scan more patients with minimal use of resources. “Innovation (on the Prodiva) is mostly on the workflow,” he said.
The value-oriented scanner, which is pending FDA clearance, features a simplified and guided patient setup. It offers Philips’ dStream digital broadband technology and the company’s In-bore Connect, a version of Philips' Ambient Experience, which presents potentially calming visual images into the bore of the magnet as it reduces acoustic noise.
Siemens showcased its 3T Vida, a 70 cm-bore scanner (introduced in March 2017 at the European Congress of Radiology) and Terra, the first 7T MR scanner cleared by the FDA. The higher magnetic field of the Terra can improve visualization. But its field strength caused the FDA to restrict marketing only for scans of the head and extremities. And even then the FDA restricts marketing the unit for use only on patients weighing no less than 66 pounds.
After GE showed about a half dozen work-in-progress MR scanners at RSNA 2016, a key takeaway point in the MR section of the company’s booth was that “now you can actually buy every one of them,” said Michael Brandt, CMO of GE’s global MR business. The company has released eight new MR scanners in four years, he said.
CT Reaches New Technology Milestones
Toshiba added to its reputation at the premium end of CT with the Aquilion Precision, a work-in-progress pending 510(k) clearance, that the company said can visualize structures 150 microns across – about the thickness of a human hair. Toshiba credited the new detector, which generates pixels 0.25 mm on each side, a specially made X-ray tube, gantry and advanced reconstruction technologies that allow 1,024 and 2,048 matrices.
“These are the building blocks for Aquilion in the future,” said Timothy Nicholson, senior manager of Toshiba market development for CT.
GE unveiled a premium CT with similar specs. The Revolution Frontier, which has cleared the FDA (and is expected to begin shipping in 2Q 2018), is built around the company’s Gemstone Clarity detector and Performix HD Plus X-ray tube. The superpremium CT can visualize structures to a resolution of 0.23 mm with 25 percent less electronic noise, according to GE. It also features spectral imaging.
Meanwhile GE upgraded other scanners in its CT portfolio. Notably its flagship Revolution CT now includes volumetric spectral imaging, and enhancements that promise to improve scan and reconstruction.
Philips debuted its IQon Elite Spectral scanner for emergency and trauma care, as well as oncology. (A radiation therapy planning couch is included; the visualization of bone marrow pathology is optimized, according to the company.) Like its predecessor, this latest version of the IQon records X-rays with energies across the CT spectrum. The IQon Elite also has faster reconstruction. The company speculates that as many as 200 patients could be scanned over 16 hours.
Siemens extended the concept of patient-centric mobile workflow with two new members of its “go” family – the Somatom go.All (64 slices per rotation) and go.Top (128 slices per rotation). Each uses tablet and remote controls, as do the two versions of the family unveiled at RSNA 2016. This allows Siemens’ “one-room concept,” which puts technologists into closer and more prolonged contact with patients.
The German company is positioning the two scanners for sale into emergency medicine, interventional radiology and cardiology. (Siemens considers cardiology to be a major growth area due to a sharp rise in the popularity of CT angiography.)
The 64-slice go.All can scan up to 100 mm in one second. The 128-slice go.Top can cover up to 175 mm per second with a range of 200 cm, enough to scan the length of a patient more than 6 and a half feet tall. The X-ray tube can be adjusted in 10-kilovolt increments.
The go.Top also offers TwinBeam Dual Energy imaging. This splits the X-ray beam into two energy spectra before it reaches the patient, enabling providers to simultaneously examine the same body region at two different energy levels.
Patient-Centric Mammography
Of all the products on the RSNA exhibit floor, none had a feature more obviously focused on the patient than GE’s Senographe Pristina mammography system. The company’s new Dueta feature, which cleared the FDA in September, addresses a long-standing complaint of women by putting breast compression literally in their hands.
“We are making women an active part of the experience,” said Agnes Berzsenyi, president of GE women’s health.
In routine diagnostic use, the technologist using Pristina Dueta positions the breast; sets a minimum level of compression; then turns control over to the patient, who uses the Dueta wireless remote to further compress the breast.
In a survey of 315 European women, 80 percent of those who used Dueta said it improved comfort. Ironically, women often compressed the breast more than a technologist would likely do, Berzsenyi said.
Adding to the comfort are a thin X-ray detector and rounded edges on the Pristina breast table against which patients lean, she said
Siemens chose a different route to improving patient comfort on its premium Mammomat Revelation. The company at RSNA 2017 unveiled a breast biopsy solution for the tomosynthesis platform, one that integrates a specimen imaging tool.
Samples are usually imaged on a second system or on a dedicated specimen scanner located away from where the mammograms are taken. That doesn’t have to happen, when using the Revelation, according to the company. With the integrated breast biopsy solution onboard the FDA-pending Mammomat Revelation, samples can be visualized at the technologist workstation within 20 seconds. The patient is not exposed to additional radiation. This improves workflow, shortens patient compression time, and improves the patient experience, according to the company.
Other Notables at RSNA 2017
At RSNA 2017, Siemens’ unveiled its Nexaris therapy platform. The goal of the hybridized platform, which is built around the company’s robotic angiography system Artis pheno, is to support diagnosis and intra-operative real-time imaging.
With Nexaris, multiple modalities can be applied intra-operatively without repositioning or moving the patient. This combination is designed to increase efficiency, convenience and patient safety.
Two configurations are pending FDA clearance: Nexaris Angio-CT and Nexaris Angio-MR-CT. Installation of either configuration can be done in one or more rooms. The CT component is essentially a gantry on rails, with rails that can extend between two rooms. Separate from the interventional suite, the gantry can be used for diagnostic work. Thanks to the rail system, the gantry can be called into the inteventional suite as needed. The Nexaris Angio-MR-CT uses a stationary MR scanner, either an Aera 1.5 Tesla or Skyra 3T.
Although the price of combining two or three big-ticket modalities mounts quickly, the investment will pay off for the institution in the long run, according to Andreas Fischer, head of marketing for Siemens advanced therapies. “In times of value-based reimbursements, you need to make sure that the treatment is being done when the patient gets off the table, because you (won’t be reimbursed) to treat the patient multiple times,” Fischer said.
Related RSNA 2017 Content:
Key RSNA 2017 Study Presentations, Trends and Video
Watch the VIDEO “Deep Learning is Key Technology Trend at RSNA 2017” where Freiherr and ITN Editor Dave Fornell explain how AI is being incorporated into medical imaging.
Watch the VIDEO “Key Imaging Technology Trends at RSNA 2017” with ITN Contributing Editor Greg Freiherr and ITN Editor Dave Fornell.
Watch the VIDEO “Editor’s Choice of the Most Innovative New Imaging Technology at RSNA 2017.”
Read the blog "RSNA 2017: Often Real Systems (Were) Not Available."
Watch an interview with Adam Flanders, M.D., chair of the RSNA Radiology Informatics Committee, in the VIDEO “How Utilization of Artificial Intelligence Will Impact Radiology.”
Watch a VIDEO examples from RSNA 2017 of how AI is being implemented in radiology IT products.


 April 22, 2024
April 22, 2024