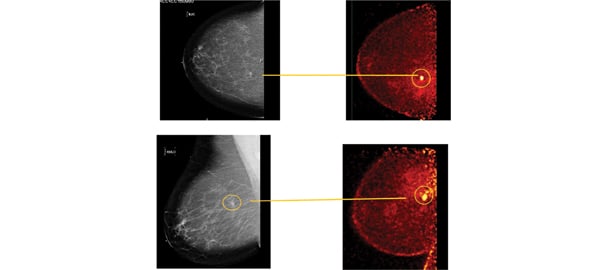
These photos compare the screening mammogram and the diagnostic PEM.
Image-guided percutaneous vacuum-assisted core biopsy plays a crucial role in the initial diagnosis and subsequent pre-surgical planning of women with breast cancer. Stereotactic X-rays, ultrasonography and magnetic resonance imaging (MRI) have been used to guide biopsies. The choice of the imaging modality used is typically based on the modality that visualizes the lesion best, the size and location of the target lesion, and equipment availability.
Functional imaging with positron emission tomography (PET) using fluorodeoxyglucose (FDG) has an advantage in that it relies on differences in cellular metabolic activity. One could then hypothesize that if this form of molecular imaging achieved high enough spatial resolution, it could be used to guide the tissue sampling and may allow for a more reliable sampling of viable tumor tissue.
Positron emission mammography (PEM), a high-resolution breast PET scanner, relies on differences in glucose metabolism to identify breast cancers from normal breast cells. Using PEM, we have an opportunity to find cancers at an even earlier stage than that detected with breast MRI, and we may even have the opportunity to find atypia because it changes cellular metabolism prior to the advent of neoangiogenesis. PEM has been shown in recently published prospective data to have similar sensitivity and superior specificity to breast MRI.
PEM Biopsy Provides Quick Feedback
The Naviscan PEM scanner is a small, compact, mobile device, similar in size to conventional ultrasound devices. It uses plates that house detectors which are able to mimic mammographic views and permit imaging in the CC and MLO projections, as well as visualization of the axilla with mere gentle immobilization. Since 2008, the Stereo Navigator PEM-guided biopsy accessory has been approved by the U.S. Food and Drug Administration (FDA). This necessary additional tool allows biopsy of lesions down to 1.3 mm, which may only be visible or accessible on PEM.
The biopsy procedure is similar to an upright stereotactic biopsy, with the patient seated with her head resting on the paddle. In our experience, this procedure seems to be well-tolerated by patients, particularly if they are claustrophobic, kyphotic or otherwise contraindicated for MRI. After the procedure is completed, specimens are imaged to confirm uptake above level of background with immediate feedback that biopsy has been successful. This is unlike MRI biopsy, where we are required to wait for final pathologic diagnosis by the pathologist to determine concordance.
A Case Study Illustrates this Approach
The following case study demonstrates the strength of this PET-guided biopsy approach. A 55-year-old post-menopausal woman presented for routine screening mammogram. Bilateral mammography showed scattered fibro-glandular densities. A new 5 mm irregular spiculated density was seen in the right breast at the 1 o’clock position, 7 cm posterior to the nipple, and evaluated as BIRADS-5, highly suspicious of malignancy. (See photos.) A targeted ultrasound failed to identify the lesion. A biopsy under stereotactic guidance was recommended.
The pathology from the stereotactic biopsy was benign glandular tissue, which was thought to be discordant. In addition, the patient suffered a large hematoma. A delayed right breast needle-localized excisional biopsy of the tissue marker was performed after resolution of the hematoma and development of two new areas of fat necrosis. Pathology yielded discordant benign results once again, but the patient remained BIRADS-5. MRI could not be performed due to the patient’s weight.
Subsequent organ-specific breast PET or PEM imaging showed a post-surgical rim of 18 F-FDG uptake consistent with granulation at the site of excisional biopsy with modest uptake of FDG. In addition, a 9 mm oval lesion of intense FDG uptake was identified contiguous with the inferior aspect of the biopsy cavity consistent with suspected malignancy. (See photo.) Two adjacent areas of mild FDG uptake were consistent in location and activity with areas of fat necrosis seen mammographically. The patient then underwent one of the first PET-guided breast biopsies as part of a clinical trial.
High-resolution breast PET-guided biopsy was performed using Naviscan’s Stereo Navigator software to target the lesion and provide direction on the best approach for vacuum-assisted biopsy (done using a Suros ATEC 9 gauge from Hologic). The four-scan biopsy process typically takes about 20 minutes.
The Stereo Navigator targets the lesion in three dimensions. Two biopsy passes were made to ensure adequate sampling. The biopsy cores were imaged on the PEM scanner and showed high levels of FDG uptake, confirming that the lesion had been accurately sampled, similar to how calcifications are imaged using stereotactic methods.
Histopathology of the core biopsy found Grade III infiltrating ductal carcinoma, finally, concordant pathology. The patient was referred for definitive surgical excision. Final surgical pathology showed residual high-grade intraductal carcinoma.
This patient’s case illustrates how PEM confirmed that the lesion seen on anatomical imaging, which was worrisome for malignancy, can be localized confidently by the high FDG uptake levels. It also demonstrates the accuracy of PEM-guided biopsy to localize, target and verify that targeted tissues have been sampled. Further, this case highlights how molecular imaging, PEM in particular, can be used to problem-solve, even when a women’s imaging center has access to stereotactic-, ultrasound- and MRI-guided biopsy approaches.
“This case beautifully shows how PEM not only identifies lesion location, but also can assist in identifying lesion pathology by assessing the quantitative uptake of FDG,” said Schilling. “In this case, we absolutely knew exactly which area of uptake corresponded to the suspected cancer. This patient, although not well suited for MRI, was successfully diagnosed using our newest tool, PEM.”
Kathy Schilling, M.D., is medical director of imaging and intervention, Women’s Center/Center for Breast Care, Boca Raton Regional Hospital, Boca Raton, Fla.


 April 18, 2024
April 18, 2024 








