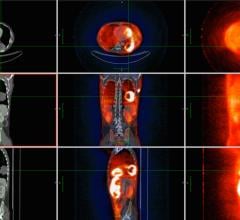
October 9, 2013 — Each year, 13 percent of all newly diagnosed lung cancer patients are diagnosed with small-cell lung cancer (SCLC). Approximately 39 percent of patients with SCLC are diagnosed with limited-stage disease. These patients are often treated with chemotherapy and definitive radiation therapy. Staging information is essential because of the high propensity for metastatic disease in SCLC, and the identification of metastases can spare patients from the toxicity associated with thoracic radiotherapy. Furthermore, in those patients who do receive radiotherapy, knowing the exact extent of disease may permit more accurate treatment volume delineation.
Until 2011, the National Comprehensive Cancer Network (NCCN) recommended a bone scan as part of the initial evaluation of all newly diagnosed SCLC patients. However, in 2012, the NCCN began recommending positron emission tomography computed tomography (PET-CT) in lieu of bone scan. Researchers from the University of Pennsylvania wanted to know the clinical impact of using PET instead.
A study published in the July issue of the Journal of Thoracic Oncology (JTO), concluded that PET-CT improves staging accuracy and intrathoracic disease identification, which translates into an improvement in clinical outcome in these patients.
"Pretreatment PET staging of LS-SCLC was associated with improved survival," reported the authors. "PET-staged patients had an improved three-year overall survival from diagnosis (47 percent versus 19 percent) compared with those with LS-SCLC who were not staged with PET." The lead author of this work was Eric Xanthopoulos, M.D. International Association fort the Study of Lung Cancer (IASLC) co-authors included Corey Langer, Charles Simone and Ramesh Rengan.
For more information: www.iaslc.org


 April 23, 2024
April 23, 2024