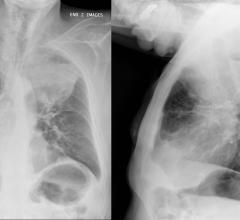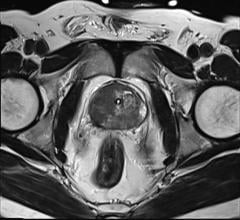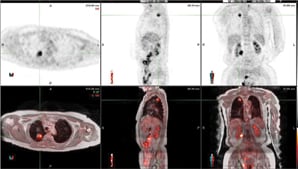
In this patient with lung cancer, MR/PET clearly demonstrates an area of activity in the right apex, shown by the cross-hairs. No other areas are identified, indicating localized disease. The top rows depict MRAC images the bottom are fused MR/PET images.
Magnetic resonance/positron emission tomography (MR/PET) is the newest imaging modality falling within the category of hybrid imaging. It sits at the crossroads of successful tradition and innovation. It is traditional in that it combines anatomical and functional molecular imaging from two potent imaging modalities; at the same time it is innovative, in that it combines the full potential of anatomic and multiparametric imaging of MRI with the molecular information of PET.
We are fortunate at Case Western Reserve University and University Hospitals Case Medical Center to have one of the first MR/PET units in the Global Advanced Imaging Innovation Center. It is also one of the first units to be placed in a predominantly clinical environment — our new Seidman Cancer Center — for the benefit of our patients. MR/PET offers multiple advantages: It can perform whole body imaging with MR’s inherent quality of superb soft tissue contrast. It also allows for radiation reduction as compared to computed tomography (CT). As a single integrated unit, MR/PET provides the ease of a simplified workflow while maintaining the integrity of both state-of-the-art components MR and PET.
Besides whole body imaging, MR/PET can achieve focused organ-based MR imaging with specific detail to abnormal areas. Thus, MR/PET provides comprehensive information in a number of clinical applications. Certainly, oncologic applications come to the fore in many body areas and specific neoplasms, including breast, rectum and prostate cancer, GYN malignancies, head and neck, soft tissue and CNS tumors.
Of particular interest is MR/PET in pediatric patients, since radiation exposure is reduced compared to PET/CT, as the CT part is obviated. Clinical areas of interest outside oncology are cardiovascular imaging, particularly atheromatous plaques and cardiac function, and neurologic applications, such as degenerative diseases, multiple sclerosis, dementia, Alzheimer’s disease and epilepsy.
There Are Some Challenges
Although MR/PET offers many opportunities, it also has its share of challenges. The integration of both technologies in one single device has been a considerable technical challenge due to a number of interferences between the two modalities. Hardware adjustments had to be made proposing avalanche photodiode technology for PET instead of photomultipliers, [1] and MR shielding had to be appropriately adjusted. [2]
Because the two components are positioned at a short distance in our unit, both parts maintain their independence. This design minimizes the interference between the two components and avoids compromises or distortions in the images obtained by either modality. The latter can be an issue in units where the PET component is within the MR magnet.
The presence of additional hardware within the PET system, such as MRI coils, cables and connectors, causes misregistration of PET counts and image artifacts. [3] Incomplete encompassing of patients in an MR image results in truncation artifacts and leads to incomplete attenuation maps with artifacts in the PET image. These issues are related to the system design and need to be compensated. Despite considerable efforts to address these problems, still a number of technical issues remain unsolved or require further optimization.
Accurate attenuation correction, a key element of quantification of PET activity, needs to be fully developed, optimized and validated in MR/PET. While attenuation correction in PET/CT is based on CT (CTAC) density, attenuation correction for different tissues in the human body based on MRI is a major challenge. MRI relies on the presence of hydrogen nuclei and tissue relaxation properties, rather than on tissue densities. Although various approaches to magnetic resonance attenuation correction (MRAC) have been proposed [4,5,6,7], to date, there is no consensus on a best approach.
MR/PET is also a challenge for the current social economic environment, where high technology is questioned, particularly if outcomes are not yet proven. The high costs of these hybrid units and their doubtful financial viability require caution and thoughtful use. Although this technology is born with many opportunities, its applications with most beneficial outcomes are still unclear.
Study length is another issue, considering that both MRI and PET scans are time-consuming tests. Clinically viable MR/PET units will require intelligent design of time-efficient MRI protocols. Facing the intrinsic complexity of MRI instead of the simplicity of CT will require collaboration between nuclear medicine and MR specialists to master aspects of operational practicality. The establishment of integrated teams, including technologists trained in both MRI and PET, basic scientists, clinicians other than radiologists and other professionals, will be essential to fully exhaust the potential of this new modality.
Early Experiences With the Technology
Having an MR/PET unit in the Advanced Global Imaging Innovation Center offers us an opportunity to provide patients with the benefits of this new technology. The center is the result of a large grant by the state of Ohio, Philips and our hospital and medical school. (See sidebar below for more information.)
Installation, which was according to appropriate specifications for both MR (Faraday cage) and PET (lead-lined room), and implementation in a new building (the University Hospitals Seidman Cancer Center) took more than a year. Distinct attention was paid to assembling our team of basic scientists, nuclear bioengineers and physicists, radio-chemists and radiopharmacists, nuclear medicine physicians, radiology experts in MRI in several body areas, as well as research fellows in order to pave the ground for scientific activities and outstanding patient care.
Our experience with the first clinical images produced in early 2012 has been exciting. The focus of our initial scientific work is to validate MRAC and to compare it with the well established CTAC. The immediate vicinity of our MR/PET and PET/CT units in the center, next to each other and with identical PET components, facilitates this important comparison. In-depth evaluation of clinical indications is planned for the near future.
We are convinced that MR/PET will become a key modality within the current armamentarium of imaging technology which we have at our fingertips.
Pablo R. Ros, M.D., M.P.H., Ph.D., is with the Department of Radiology, University Hospitals Seidman Cancer Center and Case Medical Center at Case Western Reserve University.
References:
1. Judenhofer MS, Catana C, Swann BK, Siegel SB, Jung WI, Nutt RE, Cherry SR, Claussen CD, Pichler BJ. “PET/MR images acquired with a compact MR-compatible PET detector in a 7.0T magnet.” Radiology. 2007; 244(3):807-14.
2. Hofmann M, Pichler B, Schölkopf B, Beyer T. “Towards quantitative PET/MRI: a review of MR-based attenuation correction techniques.” Eur J Nucl Med Mol Imaging. 2009; 36 Suppl 1:S93-104.
3. Delso G. Martinez-Moller A. Bundschuh RA. Ladebeck R. Candidus Y. Faul D. Ziegler SI. “Evaluation of the attenuation properties of MR equipment for its use in a whole-body PET/MR scanner.” Physics in Medicine & Biology. 55(15):4361-74, 2010
4. Malone IB, Ansorge RE, Williams GB, Nestor PJ, Carpenter TA, Fryer TD. “Attenuation correction methods suitable for brain imaging with a PET/MRI scanner: a comparison of tissue atlas and template attenuation map approaches.” J Nucl Med. 2011;52(7):1142-9.
5. Hofmann M, Bezrukov I, Mantlik F, Aschoff P, Steinke F, Beyer T, Pichler BJ, Schölkopf B. “MRI-based attenuation correction for whole-body PET/MRI: quantitative evaluation of segmentation- and atlas-based methods.” J Nucl Med. 2011 Sept;52(9):1392-9. Epub 2011 Aug 9.
6. Hofmann M, Steinke F, Scheel V, Charpiat G, Farquhar J, Aschoff P, Brady M, Schölkopf B, Pichler BJ. “MRI-based attenuation correction for PET/MRI: a novel approach combining pattern recognition and atlas registration.” J Nucl Med. 2008 Nov;49(11):1875-83. Epub 2008 Oct 16
7. Salomon A, Goedicke A, Schweizer B, Aach T, Schulz V. “Simultaneous reconstruction of activity and attenuation for PET/MR.” IEEE Trans Med Imaging. 2011 March;30(3):804-13. Epub 2010 Nov 29.
SIDEBAR:
Imaging Innovation Center Offers Latest Technology
The Philips Healthcare Global Advanced Imaging Innovation Center, based at University Hospitals Case Medical Center (UHCMC) in Cleveland, was formed in 2010 as a partnership between UHCMC, Case Western Reserve University and Philips Healthcare. It was developed to support the creation and testing of new imaging agents and technologies as well as provide researchers access to the latest advanced technologies. As part of the center, the latest Philips Healthcare imaging equipment is developed in Cleveland and validated for clinical efficacy and product release. Philips Healthcare’s global computed tomography (CT) and nuclear medicine headquarters also is in Cleveland. The center, which received $2.5 million in research grants last year from the Ohio Third Frontier Commission to advance its work, further establishes the Cleveland area as a worldwide hub for imaging technology, bringing together radiologic experts to coordinate clinical research, education, development and commercialization of advanced imaging technologies.
The center provides the latest imaging technologies to benefit many areas, including cancer, neurologic conditions, cardiovascular disease, orthopedic, gastrointestinal diseases and more, while providing Philips Healthcare as well as scientists and physicians from Case Western Reserve University and UHCMC the opportunity to create synergy and bring new technologies to patients more quickly. It houses the latest Philips imaging equipment, including: a positron emission tomography/magnetic resonance imaging (PET/MRI) machine; a new-generation PET/computed tomography (CT) scanner; a new diagnostic PET scanner, a CT system and a 3.0 T intra-operative MR unit.
“The collaboration between our various organizations will create a pipeline to move innovative technologies more quickly into patient care,” said Thomas F. Zenty III, CEO of University Hospitals, when the project was first announced. “We are pleased to have this center based at University Hospitals Case Medical Center and our new cancer hospital, knowing that it will lead to patients receiving better imaging for more accurate diagnoses of their cancer, heart disease and neurologic conditions.”
Case Western Reserve University School of Medicine is the largest medical research institution in Ohio. The school’s primary affiliate is UHCMC. University Hospitals serves the needs of patients through an integrated network of hospitals, outpatient centers and primary care physicians. UHCMC is the core of the health system. It is home to prestigious clinical and research centers including cancer, pediatrics, women’s health, orthopedics and spine, radiology and radiation oncology, neurosurgery and neuroscience.
For more information, visit www.uhhospitals.org


 April 23, 2024
April 23, 2024