Image courtesy of IBA
According to the latest statistics from the American Cancer Society (ACS), almost 165,000 new cases of prostate cancer were expected in 2018, with over 29,000 men expected to die from the disease this year.
Radiation dose management is particularly important for prostate cancer treatment. The prostate is adjacent to several radiosensitive organs, including the rectum and the bladder. Overexposure of these adjoining areas can lead to detrimental side effects such as loss of sexual function, bladder control issues and rectal bleeding.
New technologies and techniques have evolved in recent years to target dose more specifically to the cancer, improving both disease control and patient quality of life.
Brachytherapy
One method that allows more precise deposition of treatment radiation is brachytherapy, which implants radioactive seeds directly into the tumor. There are two types of brachytherapy:
- In low-dose-rate (LDR) brachytherapy, seeds are permanently implanted inside the tumor. The seeds are designed to emit low doses of radiation over an extended period of time; and
- High-dose-rate (HDR) brachytherapy inserts the seeds temporarily, emitting large doses of radiation in a short period.
A national study published in the Journal of the American Medical Association (JAMA) in March compared the effectiveness of three different treatment approaches for prostate cancer: surgery only, external beam radiotherapy (EBRT) only (with androgen deprivation therapy), and EBRT plus brachytherapy. The retrospective study looked at 1,800 cases of very high-risk prostate cancer patients, divided approximately equally between the three groups. The primary endpoint for gauging effectiveness was prostate cancer-specific mortality; secondary endpoints were distant metastasis-free survival and overall survival. Combining EBRT and brachytherapy dropped mortality to 3 percent (versus 12 and 13 percent, respectively, for surgery and EBRT alone). Even more significantly, metastatic cancer rates plummeted from 24 percent in groups one and two, to just 8 percent in group three.1
“Compared to other approaches, the combination of external beam and brachytherapy showed dramatic improvements in cancer mortality and metastatic disease rates — something radiation oncologists at Beaumont have suspected for some time,” said Daniel Krauss, M.D., radiation oncologist and principal investigator at the Beaumont, Royal Oak (Michigan) research site.
IsoRay introduced a new brachytherapy device specifically for prostate cancer applications in June. The Build-Blu delivery system is a disposable seed stranding device that allows users to build custom-configured strands with cesium (Cs)-131 in the operating room.
Cesium-131 has the shortest half-life of the isotopes used for prostate brachytherapy at just 9.7 days. According to IsoRay, which is the only manufacturer of Cs-131 seeds, they deliver 90 percent of the total dose in just 33 days, compared to 58 days for palladium (Pd)-103 and 204 days for iodine (I)-125. Clinical research suggests the short half-life of Cs-131 leads to the cessation of urinary-related side effects within six months of treatment completion.2
Perhaps the greatest benefit of the Build-Blu system is that it allows proceduralists to visualize the patient’s anatomy and adapt treatment in real time. The results of a 13-year retrospective study published in 2013 suggested that clinical outcomes, measured through biochemical no evidence of disease (bNED), were greatly superior with real-time intra-operative planning of prostate cancer brachytherapy compared to preoperative planning alone
(95 percent versus 67 percent).3 All patient cases in the study utilized I-125.
Proton Therapy
Proton therapy has been gaining clinical traction in recent years as a less harsh alternative to traditional photon-based radiation therapy. In proton therapy treatments, the radiation beam conforms more closely to and does not pass beyond the target, sparing adjacent healthy tissue and organs. This has been shown to preserve organ function and potentially reduce harmful side effects of treatment.
Clinical research has explored the use of proton therapy on cancers in several highly sensitive areas of the body, including the esophagus, head and neck cancers, as well as the prostate. Two studies presented at the 2017 Particle Therapy Cooperative Group — North America (PTCOG-NA) annual meeting in October compared the clinical outcomes for prostate cancer patients treated with proton therapy versus intensity modulated radiation therapy (IMRT).
In the first study, researchers from the Northwestern Medicine Chicago Proton Center and the University of Florida Health Proton Therapy Institute compared outcomes using Surveillance and End Results Registry (SEER) data from the National Institutes of Health National Cancer Institute (NCI) and Medicare claims data. All patients had received proton therapy between 2006 and 2012. The research team evaluated treatment side effects using Medicare billing codes for:
• Gastrointestinal (GI)
• Genitourinary (GU)
• Endocrine; or
• “Other” complications.
Results showed the proton therapy group had a higher five-year survival rate than the IMRT group (94 percent to 87 percent), with fewer bladder complications and secondary cancers for the former.4
The second study, out of The University of Florida Health Proton Therapy Institute and Mayo Clinic Arizona, directly compared clinical outcomes between PT and IMRT patients. The research team found similar complication rates between the two groups, but low- and intermediate-risk prostate cancer patients saw improved disease control with proton therapy.5
Spacer System Enhances Patient Safety
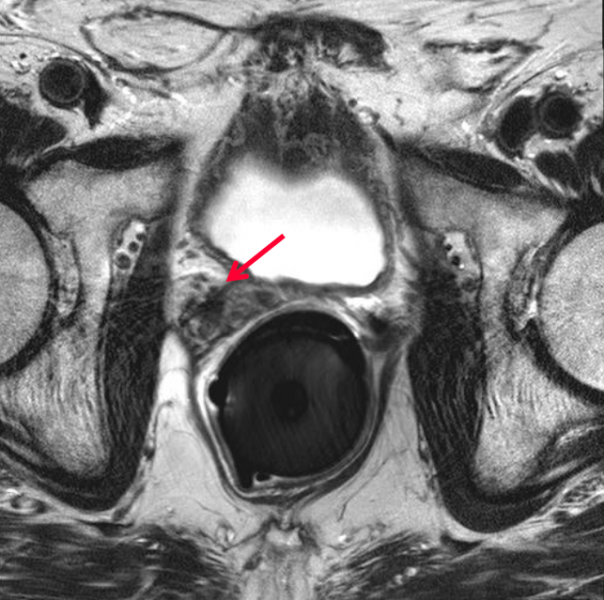
Regardless of the type of radiotherapy delivered, prostate cancer treatment requires high doses of radiation to achieve disease control. One important consideration during dose calculation is the position of the rectum, a portion of which often remains in the target treatment area. One strategy that has been employed in the past to protect the rectum and other radiosensitive areas is to create physical separation between the prostate and the rectum through use of a spacer. Multiple products and materials have been used in this capacity, the most recent of which is a hydrogel-based product that dissolves following treatment completion.
Massachusetts-based company Augmenix received U.S. Food and Drug Administration (FDA) clearance for the SpaceOAR System in early 2015. The hydrogel is administered as a liquid and solidifies into a soft gel that temporarily positions the anterior rectal wall away from the prostate. Following the treatment, the gel liquefies and is absorbed and excreted from the body in the patient’s urine.
Clinical trial results reported in the last 18 months suggest the SpaceOAR System has been successful in limiting poor outcomes over the long term. Three-year post-treatment data was published in the Red Journal in January 2017 evaluating:
• Radiation dose to critical structures (prostate, rectum, bladder, penile bulb);
• Rectal and urinary toxicity; and
• Patient quality-of-life (QOL) impact.
Results showed a 73.5 percent rectal V70 dose reduction during treatment, along with a 49 percent reduction in median penile bulb dose compared to the control group treated without SpaceOAR. At three years post-treatment, SpaceOAR patients exhibited a 78 percent reduction in late rectal toxicity complications; no SpaceOAR group patients experienced grade two or worse rectal toxicity, compared to 5.7 percent of the control group.6
From the quality of life perspective, 2.5 percent of the experimental group experienced clinically significant declines in all three QOL areas — bowel, urinary and sexual — compared to 20 percent in the control group. Augmenix announced the start of a new SpaceOAR trial in June that will assess a technique to spare nerve bundles and arteries involved in sexual function.
The POTEN-C trial will experiment with delivering dosage only to the cancerous portion of the prostate via stereotactic ablative radiotherapy (SABR), while SpaceOAR will be used to separate the prostate and rectum. Half of the patient population will be treated with the new technique, while the other half will receive standard SABR.
“Nowadays, mortality after treatment for localized prostate cancer is as low as 1 percent at 10 years,” said Neil Desai, M.D., assistant professor of radiation oncology and Dedman Family Scholar in Clinical Care at UT Southwestern Medical Center, and principal investigator of the POTEN-C trial. “By contrast, as many as half of all patients being treated for prostate cancer will experience some decline in sexual function. It is appropriate, therefore, that our focus has shifted to this aspect of quality of life.”
A total of 120 patients will be enrolled in the POTEN-C trial at up to nine major medical center sites across the United States. All patients will be followed for two years.
References
1. Kishan A.U., Cook R.R., Ciezki J.P., et al. Radical Prostatectomy, External Beam Radiotherapy, or External Beam Radiotherapy With Brachytherapy Boost and Disease Progression and Mortality in Patients With Gleason Score 9-10 Prostate Cancer. Journal of the American Medical Association, March 6, 2018. doi:10.1001/jama.2018.0587
2. DeFoe S.G., Beriwal S., Smith R., Benoit R. Is There Decreased Duration of Acute Urinary and Bowel Symptoms After Prostate Brachytherapy With Cesium 131 Radioisotope? International Journal of Radiation Oncology Biology Physics, Sept. 1, 2008. https://doi.org/10.1016/j.ijrobp.2008.06.1093
3. Matzkin H., Chen J., German L., Mabjeesh N.J. Comparison between preoperative and real-time intraoperative planning 125I permanent prostate brachytherapy: long-term clinical biochemical outcome. Radiation Oncology, Dec. 17, 2013. https://doi.org/10.1186/1748-717X-8-288
4. Hartsell W., Bentefour, Dooling, et al. Proton therapy is associated with superior survival and decreased risk of complications compared to IMRT for intermediate risk prostate cancer: A Medicare/SEER database study. Presented at PTCOG-NA Annual Meeting, Oct. 23-25, 2017.
5. Mendenhall, Wong, Bryant, et al. Potential Improved Outcomes with Proton Therapy in Prostate Cancer: A Comparison of IMRT and Proton Cohorts. Presented at PTCOG-NA Annual Meeting, Oct. 23-25, 2017, of a Phase III Trial. International Journal of Radiation Oncology Biology Physics, April 1, 2017. https://doi.org/10.1016/j.ijrobp.2016.12.024


 April 18, 2024
April 18, 2024