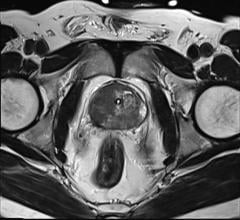
August 5, 2014 — GE Healthcare announced its first integrated, simultaneous, time-of-flight (TOF) capable, whole-body Signa PET/MR (positron emission tomography/magnetic resonance) is 510(k) pending at the U.S. Food and Drug Administration (FDA).
Powered by simultaneous image acquisition from GE’s latest 3.0T MR and PET technologies, the Signa PET/MR helps clinicians achieve improved scan efficiency that may lead to more effective treatment paths for clinicians to offer their patients, particularly for oncology, neurology and cardiology.
MR is ideal for imaging soft tissue as well as functional and morphological details, while PET enables clinicians to visualize cellular activity and metabolism. When these two tools are combined, clinicians may be able to see early cellular changes that can be accurately mapped onto MR images. With this knowledge, clinicians may be able to shorten the time between diagnosis and treatment, in addition to offering the convenience of simultaneous PET and MR scans for patients. Research systems are installed at Stanford University, University of California San Francisco and University of Zurich.
“We are excited about Signa PET/MR because of its clinical and research potential,” said Andrei H. Iagaru, M.D., associate professor of radiology and co-chief of the division of nuclear medicine and molecular imaging at Stanford University Medical Center. “We have been using the system for research, and we are able to explore novel things in areas like neurology and oncology, as well as in pediatrics in the future. Additionally, it’s more convenient for the patient due to simultaneous scanning. We can also initiate innovative, complex research; simultaneity allows us to do functional neuro imaging, cardiac imaging and musculoskeletal imaging that we haven’t been able to do before. TOF offers improved image quality in PET/MR and with the increased detector sensitivity, it may lead to future improvements in dose reduction.”
The Signa PET/MR features GE’s new, exclusive MR-compatible silicon photomultiplier detector (SiPM) technology. This new digital detector is characterized by its enhanced sensitivity; it is up to three times more sensitive than conventional PET technology. It also features fast coincidence timing resolution enabling TOF reconstruction. With TOF reconstruction, the arrival times of each coincident pair of photons are more precisely detected, and the time difference between them is used to localize the PET signal accurately. TOF leads to improved PET image quality with higher structural detail and improved signal-to-noise ratio. The Siga PET/MR is designed to be fully upgradable from a Discovery MR750w 3.0T.
“We have received extremely positive feedback from our installations of the research PET/MR systems,” said Richard Hausmann, president and CEO of GE Healthcare MR. “Our research partners are very excited by the performance of the system and the potential of this new technology. We are proud to bring the first TOF-capable, simultaneous PET/MR system, pending FDA clearance, to market.”
The system is not available for sale in the United States and has not yet received CE mark. It is not available for sale in all regions.
For more information: www.gehealthcare.com


 April 17, 2024
April 17, 2024