Magnetic resonance imaging (MRI) has emerged as a valuable imaging modality for breast cancer detection and staging. Dynamic contrast-enhanced MRI (DCE-MRI) is the most sensitive technique for screening high-risk women and for evaluating the extent of disease in patients with a recent diagnosis of breast cancer.[1,2] Despite its numerous advantages, the moderate specificity of DCE-MRI can result in a substantial number of false positive findings that translate to high recall rates and unnecessary biopsies.
Reducing the number of false positives of breast DCE-MRI could substantially decrease morbidity for patients. According to results of our study recently published in Radiology, incorporating diffusion-weighted imaging (DWI) into conventional breast MRI examinations may decrease false positive findings in breast MRI and reduce preventable biopsies.
DWI is a short, noncontrast MRI sequence that has strong potential to increase specificity as an adjunct to conventional breast MRI protocols.[3-7] DWI measures the apparent diffusion coefficient (ADC), which characterizes the mobility of water molecules in vivo and indirectly reflects tissue cellularity, microstructural characteristics and membrane integrity.[8] DWI has been used successfully in neuroimaging applications, but until recently technical challenges limited implementation for imaging of other body sites outside the brain. Promising initial findings from DWI studies of the breast demonstrated significantly lower ADC measures for breast carcinomas than for benign breast lesions or normal tissue.[3-5] Furthermore, recent studies have shown improvements in breast MRI accuracy achieved through a combination of DWI and DCE-MRI features, and have identified potential ADC thresholds for differentiating benign from malignant lesions.[6,7 ]
However, there is substantial overlap between ADC values reported for benign and malignant lesions, and in particular a large fraction of nonmalignant lesions with low ADC values (overlapping with malignancies), which presents challenges for implementing a useful diagnostic ADC threshold. Breast DWI studies to date have included relatively limited numbers and/or subtypes of nonmalignant lesions, and little is known about the ADCs of specific histologic subtypes.
In this study we aimed to characterize ADC values for a variety of nonmalignant lesion subtypes representative of the false-positive findings typically encountered on DCE-MRI. We further sought to determine which particular subtypes exhibit ADC values below a previously proposed diagnostic ADC threshold and comprise the overlap with malignancies reported by previous studies.
Study Design
We retrospectively evaluated DWI characteristics for breast MRI false-positive lesions identified at our institution from October 2005 to December 2008, defined as suspicious lesions detected on MRI that were subsequently biopsied and determined to be benign. A total of 175 nonmalignant lesions in 161 women (median age, 48 years; range, 27–77 years) were included in the analysis. Clinical indications for the MRI exams were primarily to evaluate the extent of disease in patients with newly diagnosed breast cancer (51 percent) and high-risk screening (40 percent). All MRI scans were performed on a 1.5 Tesla system using a dedicated eight-channel bilateral breast coil. DWI was performed after the DCE-MRI acquisition using a DWI echo-planar imaging sequence with parallel imaging and fat suppression, and b-values of 0 and 600 s/mm2. ADC values of the 175 lesions were calculated and compared between nonmalignant subtypes and with an ADC threshold of 1.81 × 10-3 mm2/s (determined in a prior study to achieve 100 percent sensitivity). In addition, the mean ADC values of benign and high-risk subtypes were compared with malignant lesions in the previous study, which included 15 invasive ductal carcinomas, five invasive lobular carcinomas and 11 cases of ductal carcinoma in situ.[6]
DWI Characteristics of DCE-MRI False-Positive Breast Lesions
The most common DCE-MRI false-positive histologic subtype was found to be fibroadenoma (n=30), followed by atypical ductal hyperplasia (ADH; n=23), adenosis (n=21), and focal fibrosis (n=19). Maximum lesion size, as defined with dynamic contrast-enhanced MRI, ranged from 0.2 to 9.1 cm, with a median size of 1.1 cm. Lesion types included 92 (53 percent) masses, 78 (45 percent) non-masses, and five (3 percent) foci. A total of 28 (16 percent) lesions were
high-risk subtypes including ADH or lobular neoplasia.
A statically significant difference in mean ADC among subtypes was identified. ADH demonstrated significantly lower and fibroadenoma demonstrated significantly higher ADC compared to other nonmalignant subtypes. ADC values for high-risk lesions in general were significantly lower than benign lesions. Comparison with ADC values of malignant lesions (obtained in a prior study of suspicious MRI-detected lesions) showed a trend of decreasing mean ADC from benign to high-risk to malignant lesion types.
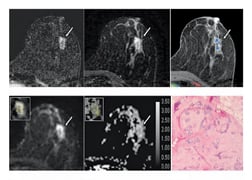
Of the 175 benign and high-risk false-positive lesions found with DCE-MRI, 81 (46 percent) had ADCs above the diagnostic threshold. The most common lesion subtypes with ADCs above the threshold were fibroadenoma (19 of 30 cases, or 63 percent), Figure E1; adenosis (11 of 21 cases, or 52 percent); and focal fibrosis (10 of 19 cases, or 53 percent). That total comprised approximately half of the lesions with ADCs above the threshold. By comparison, the most common lesion subtypes with ADCs below the threshold were ADH (18 of 23 cases), fibroadenoma (11 of 30 cases) and adenosis (10 of 21 cases). These accounted for approximately 41 percent of lesions with ADCs below the threshold.
Key Findings
In summary, our findings show promise for using DWI to improve the specificity of breast MRI. The key finding of the study is that based on a diagnostic ADC threshold, DWI helped accurately characterize as benign 46 percent of the DCE-MRI false positive lesions. Biopsy could have potentially been avoided for these lesions, which would have resulted in substantial savings in time, expense and patient discomfort, particularly as 62 of the 81 above-threshold lesions required the more costly and time-intensive MR-guided biopsy procedure. In addition, the ability of DWI to differentiate high-risk lesions requiring additional workup from other nonmalignant subtypes may further improve patient management. Assessing ADC along with DCE-MRI features may decrease false-positive MRI findings and contribute to improved radiology-pathology concordance for particular nonmalignant lesion subtypes undergoing biopsy. As for the next step, we are planning to conduct a multicenter trial to validate the findings and determine how to best incorporate ADCs into clinical breast MRI interpretations.
Sana Parsian, M.D., is currently a clinical research fellow in the department of radiology at the University of Washington, working under the guidance of Savannah Partridge, Ph.D. She has been involved with research projects ranging from utility of diffusion-weighted MRI (DWI) for improved loco-regional staging and identification of unique MRI biomarkers predictive of tumor aggressiveness. Parsian is an active member of Radiological Society of North America and plans to enter a radiology residency program in the fall.
Savannah C. Partridge, Ph.D., is a research associate professor in radiology at the University of Washington. Her background is in medical imaging physics with over 15 years of experience in development of acquisition sequences and analysis software tools for clinical and translational breast magnetic resonance imaging (MRI) applications. She is an active member of several professional societies including the Radiological Society of North America and International Society of Magnetic Resonance in Medicine, and the ACRIN-ECOG multi-center trial network breast committee. She serves as a scientific reviewer for a number of research funding organizations including the NIH, Komen and DOD Breast Cancer Research Program. A primary focus of her work is the investigation of diffusion-weighted MRI (DWI) to improve breast cancer detection and diagnosis.
In reference to our recent publication:
Parsian S, Rahbar H, Allison KH, DeMartini WB, Olson ML, Lehman CD, Partridge SC. “Nonmalignant breast lesions: ADCs of benign and high-risk subtypes assessed as false-positive at dynamic enhanced MR imaging.” Radiology 2012; 265(3): p.696-706 [Epub ahead of print] (PMID: 23033500).
References
1. Lehman CD, Isaacs C, Schnall MD, et al. “Cancer yield of mammography, MR, and US in high-risk women: prospective multi-institution breast cancer screening study.” Radiology 2007; 244(2):381–388.
2. Berg WA, Gutierrez L, NessAiver MS, et al. “Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer.” Radiology 2004; 233(3):830–849.
3. Guo Y, Cai YQ, Cai ZL, et al. “Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging.” J Magn Reson Imaging 2002; 16(2):172–178.
4. Woodhams R, Matsunaga K, Kan S, et al. “ADC mapping of benign and malig- nant breast tumors.” Magn Reson Med Sci 2005; 4(1):35–42.
5. Rubesova E, Grell AS, De Maertelaer V, Metens T, Chao SL, Lemort M. “Quantitative diffusion imaging in breast cancer: a clinical prospective study.” J Magn Reson Imaging 2006; 24(2):319–324.
6. Partridge SC, DeMartini WB, Kurland BF, Eby PR, White SW, Lehman CD. “Quantitative diffusion-weighted imaging as an adjunct to conventional breast MRI for improved positive predictive value.” AJR Am J Roentgenol 2009; 193(6):1716–1722.
7. Ei Khouli RH, Jacobs MA, Mezban SD, et al. “Diffusion-weighted imaging improves the diagnostic accuracy of conventional 3.0-T breast MR imaging.” Radiology 2010; 256(1): 64–73.
8. Le Bihan D, Turner R, Douek P, Patronas N. “Diffusion MR imaging: clinical applications.” AJR Am J Roentgenol 1992; 159(3): 591–599.


 April 16, 2024
April 16, 2024