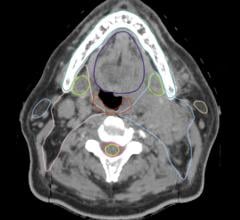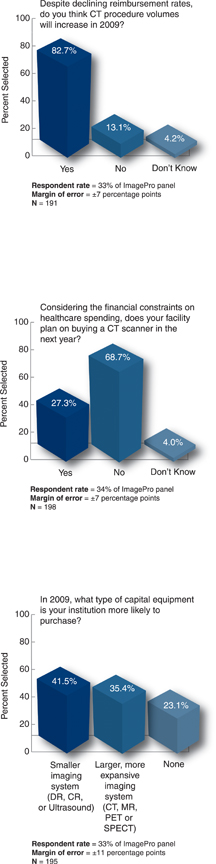
The majority of respondents believe that CT volumes will increase in 2009. Most facilities do not plan to purchase CT scanners in the next year. The difference between smaller imaging systems and larger imaging systems is not statistically significant.
Many experts lauded multidetector computed tomography (MDCT) and cardiac computed tomography angiography (CCTA) as disruptive technologies that would abruptly alter the standard of care for those with coronary artery disease. Now, years later, CCTA is slowly gaining acceptance in the medical community, both by providers, payers and administrators. Why so slowly? Perhaps the answer is simply timing. What are the hurdles? The ones that play a role include cost, radiation safety, the economy and the need for evidence-based clinical outcomes. Cost of Healthcare In the last decade, the cost of healthcare in the U.S. and especially medical imaging has skyrocketed. Expensive diagnostic exams became a target for cost containment, which would explain the non-coverage determinant for CCTA by CMS in December 2007, and the ensuing coverage confusion that followed. CMS and the insurance industry simply will not support the use of CCTA as another layer of cost. Any medical center or practice interested in developing a cardiac imaging program adopted a conservative approach. At the date of this writing, all local Medicare intermediaries and most commercial payers do reimburse for CCTA. However, reimbursement is based on Local Coverage Determinants that vary greatly in the details, and it is the attention to these details that determines appropriate reimbursement. CMS has solicited requests for input into CPT code development, but until there is a clear demonstration that CCTA is cost effective and adds value, we still face the cost restraints that are in place. Radiation Safety Secondary effects of radiation exposure are top of mind in the medical community and the general public. Several peer review studies and editorial reviews have highlighted the risks of inadvertent over-utilization of CT scanning, especially in the younger population. To address this issue, the ACR has adopted the “Image Gently” program, and manufacturers have developed dose reduction strategies. Excellent articles like those from Mayo, et al, (AJR) have delineated the risks when imaging is appropriately performed. We will need to continually monitor this issue and ensure that the appropriate utilization of MDCT prevails. Economic Recession Obviously, no one predicted the sudden global collapse of the economy last year. This has further limited growth of CCTA programs. Capital budgets have been slashed, purchases put on hold and FTEs dismissed. Systems must be efficient and, at the very least, cost neutral or they face the piper. While this is no time for expansion into an arena with questionable reimbursement, fortunately, recent studies have demonstrated cost savings when CCTA is used appropriately. For example, in the ED setting, a negative CCTA allows for safe and rapid discharge, saving a great deal of expense in unnecessary admissions and time of stay. In the office setting, those patients with a confusing presentation can be carefully evaluated with CCTA, reducing the number of more invasive and expensive catheterizations. Need for Evidence-Based Clinical Outcomes The recent National Forum on “Appropriate Coverage of Coronary CT Angiography,” organized and sponsored by the SCCT, brought together influential providers and payers to discuss the issues currently surrounding CCTA usage. Payers perceive a lack of evidence that CCTA improves clinical outcomes. This exam must demonstrate this benefit before reaching acceptance. But how do we measure an improved outcome? There are no definable metrics in place, but rapid and safe discharge, decreased utilization of downstream imaging, and modified patient behavior are being studied. Prospective randomized multi-institutional studies are expensive (especially in this economy) and time-consuming, but must be performed and must confirm these benefits. So while we must continue to cross these hurdles, the one positive aspect is that they have brought together many groups in a collegial and cooperative way in an effort to educate our industry, medical colleagues and our patients to the potential benefits of this wonderful new capability. It is up to us to jump these hurdles as quickly as possible. For more information: The MarkeTech Group



 April 16, 2024
April 16, 2024