The digital breast tomosynthesis (DBT) market has evolved rapidly within the past year, with new vendors entering the market and recent clinical data continuing to support its use. In addition, the Centers for Medicare and Medicaid Services (CMS) created dedicated reimbursement codes for DBT procedures earlier this year. MD Buyline rarely sees purchases for mammography systems that are not at least upgradable to 3-D capabilities. With the market as a whole predicted to grow by 18.1 percent through 2019, we expect the demand for this breakthrough technology only to rise.1
Evolution from 2-D to 3-D Mammography
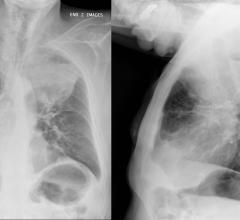
The current gold standard in breast cancer screening is two-dimensional (2-D) mammography. Although 2-D full-field digital mammography (FFDM) is successful in identifying a large number of cancers, this method has its limitations. A traditional 2-D mammography study consists of two images per breast — craniocaudal (CC) and mediolateral-oblique (MLO). The flat nature of these images can make them hard to interpret. Overlapping tissues and calcifications can also mask cancerous lesions. DBT overcomes this challenge by creating a 3-D view of the breast similar to a computed tomography (CT) scan. Radiologists can scroll through the breast, slice by slice, allowing them to see abnormalities that would have been obscured otherwise in traditional 2-D images.
This clearer view not only identifies areas that potentially would have gone undetected with 2-D mammography, but also provides greater clarity into suspicious areas that may have required a callback for additional imaging. These higher detection rates and decreased patient callbacks have made providers eager to adopt the technology.
3-D Mammography Market Overview
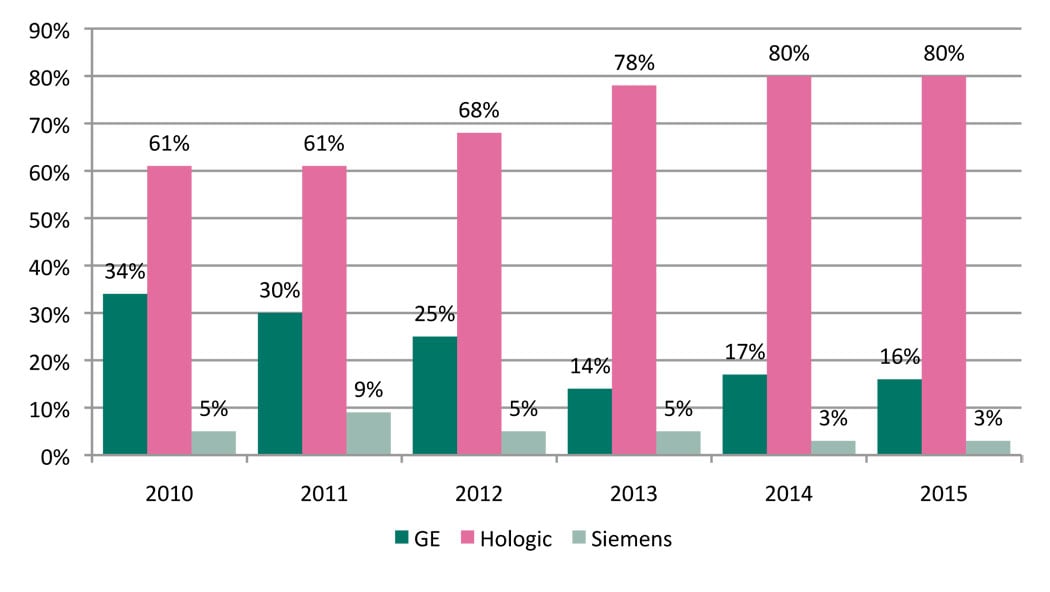
While Hologic has remained a pioneer in the DBT market, two new vendors, GE and Siemens, entered the market within the past year with add-on capabilities to their existing platforms. More recently, in July 2015, Fujifilm submitted the first module of its premarket approval application for an optional upgrade to its mammography system. (See Table 1.)
Hologic. Although Hologic has always been a strong player in the digital mammography market, its presence was further elevated when it received U.S. Food and Drug Administration (FDA) approval for its Selenia Dimensions Tomo system in 2011. At the time, it made Hologic the only vendor to offer DBT technology — a position Hologic would continue to hold for the next three years. U.S. facilities, having seen the success of DBT on an international level, were eager to begin using Hologic’s Genius 3-D technology and quickly started adding this to their portfolio of exam offerings. Although improvements in patient care played a factor in the decision to transition from 2-D to 3-D, providers also capitalized on DBT as a marketing tool to show patients that they offer the most cutting-edge technology.
Since the introduction of the Selenia Dimensions Tomo, Hologic continues to make improvements to its technology. One of the most notable advancements is C-View, a synthesized 2-D image that eliminates the need to acquire both 2-D and 3-D images. C-View allows for a shorter exam, which reduces the time a patient is under compression, and lowers radiation dose.
GE. GE received FDA approval for its digital breast tomosynthesis product, SenoClaire, in September 2014. Unlike Hologic’s tomosynthesis solution, which is only available on its newest Selenia Dimensions platform, GE designed SenoClaire as an option for its existing Senographe Essential and Senographe Care platforms.
Since Hologic introduced its 3-D system, interest in GE’s mammography products has been low, but with the introduction of SenoClaire, MD Buyline has seen increased interest from providers.
Siemens. Siemens submitted a PMA application for its DBT solution in June 2014. By April 2015, Siemens had received FDA approval for its True Breast Tomosynthesis. As with GE’s solution, Siemens also designed its offering as an upgrade to its existing Mammomat Inspiration and Mammomat Inspiration Prime platforms. As with any new introductions in the mammography market, interest has been gradual. However, we are beginning to see an increased interest in Siemens’ mammography solutions.
Purchasing Considerations for Breast Tomosynthesis Systems
Image Storage and Review. Image review capabilities and storage continue to be areas of caution when purchasing DBT solutions. Some facilities forget to consider the fact that DBT images require a significantly larger amount of storage than 2-D mammography. Because not all picture archiving and communication system (PACS) vendors are compatible with DBT, it is important to ensure that mammography and IT departments work together to create a plan for both the viewing and storage of DBT images.
Radiation Dose. When Hologic first received FDA approval for its Selenia Dimensions Tomo system, patient dose was a huge topic of conversation. Acquiring a tomosynthesis study of approximately 10-20 low-dose images in addition to the 2-D exam exposes the patient to a slightly higher radiation dose. Since then, Hologic has released its C-View option, which allows the tomosynthesis exam to be reconstructed into 2-D images, eliminating the need for additional exposures. GE’s SenoClaire 3-D MLO sequence consists of only nine exposures and delivers a dose equivalent to that of a standard 2-D MLO image. Siemens’ DBT solution is available on its Prime platform. This system features an antiscatter technology that can reduce dose up to 30 percent.
System Upgrades. Workstations must be reviewed to ensure they contain the proper software to view tomosynthesis studies. Hardware upgrades are sometimes necessary to support the larger file size associated with DBT images.
Maintaining the Competitive Edge. Although a Gallup poll from 2012 showed that most Americans are satisfied with their quality of care (82 percent), the information age is changing how patients seek this care.2 They are often better informed about their options and have a clearer understanding of the quality of care a provider can offer than ever before. Media stories and the Internet have made it exceptionally easy for patients to learn about advantages of DBT systems. As this trend continues and patients become increasingly adept at choosing their providers, hospitals will face the added challenge of competing for patients. This is a critical issue because volume is a key factor in the success of any service line.
Furthermore, when referring physicians are aware DBT is available at a certain facility, it breeds confidence in sending patients to that location. Gaining more referrals, especially in an urban area where multiple hospitals compete for the same patients, is often a huge challenge.
Customer Feedback. Although there are a lot of factors to consider when purchasing this technology, customers have reported that tomosynthesis is the best investment they have ever made. User feedback from MD Buyline’s database reports that DBT has increased business, increased cancer detection and decreased callbacks by 25-32 percent.
Clinical Evidence for 3-D Mammography
Of the many studies that have evaluated the benefits of DBT technology, none is as powerful as the Journal of American Medicine Association’s (JAMA) study released in June 2014.3 Due to the size and scope of research, which evaluated 454,850 examinations across 13 facilities, this study continues to be a major win for supporters of DBT. Results from the JAMA study showed tomosynthesis images increased the detection of invasive breast cancers by 41 percent and decreased recalls for additional imaging by 15 percent.
Another notable study was presented at the Radiological Society of North America (RSNA) annual meeting in 2014.4 Conducted in Oslo, Norway, the study looked at the single screening session of 25,547 women ages 50-69 who received either traditional 2-D imaging or 2-D imaging with DBT. Women were graded with the American College of Radiology’s Breast Imaging-Reporting and Data System (BI-RADS), which classifies breast density from 1 to 4 with 4 being the densest. The study concluded that DBT not only was valuable for women with dense breast tissue (2-4 rating), but also increased the cancer detection rate in fatty breast tissue.
More recently, results from the Malmö Breast Tomosynthesis Screening Trial were published in April 2015.5 This Swedish study compared two-view digital mammography with one-view DBT imaging in 7,500 women ages 40-74. This study concluded that one-view DBT has the potential to be a stand-alone screening modality for the detection of cancers. Currently, two-view 2-D digital mammography is the gold standard.
Reimbursement for Digital Breast Tomosynthesis
Effective Jan. 1, 2015, CMS created dedicated reimbursement codes for DBT procedures. These new codes (77063 for DBT Screening and G0279 for DBT Diagnostic) are charged in addition to the 2-D mammography charges.
Although not all insurance carriers are covering DBT exams, these new codes are the first step in insurance carrier adoption. (See Figure 1.)
Future of the 3-D Mammography Market
As more vendors enter the DBT market, hospitals will have the opportunity to make more thorough evaluations of the technology and price associated with its purchase. We have already seen GE offer customer loyalty incentives and Hologic offer promotional discounts on their DBT solutions. We anticipate this is just the beginning of a much more competitive mammography market. As facilities continue to replace older mammography equipment, we also expect to see a wider adoption of digital breast tomosynthesis.
Rachael Bennett joined MD Buyline in 2008 with seven years of clinical experience in the medical field. She is the primary clinical analyst for linear accelerators, stereotactic radiosurgery, mammography systems, biopsy systems and other radiation oncology and women’s health capital equipment codes. She currently holds registries as both a radiographer and radiation therapist through the American Registry of Radiologic Technologists (ARRT).
Katie Regan joined MD Buyline in 2013, bringing with her seven years of clinical and laboratory research experience. She previously worked as a senior research associate for DAVA Oncology, a consulting group focused on the facilitation of successful oncology drug development. At MD Buyline, she is responsible for all clinical, financial and general healthcare publishing projects.
References:
3. Friedewald et al., JAMA. 2014; 24:24999. www.ncbi.nim.nih.gov/pubmed/25058084. Accessed Sept. 30, 2015.
4. Skaane et al., Radiology Society of North America. 2014: VSBR31-16. www2.rsna.org/timssnet/rsna/media/pr2014/skaane/abstract/SkaaneAbstract.pdf. Accessed Sept. 30, 2015.
5. Lang et al., Eur Radiol. 2015; preprint. www.ncbi.nim.nih.gov/pubmed/25929946. Accessed Sept. 30, 2015.


 April 18, 2024
April 18, 2024